Growth factors
3D structure of the kinase domain of Activin receptor1 (Acvr1) complex with inhibitor shows with the protein including a and a [1]. Water molecule is shown as red sphere. Rabbit [2]. Water molecules are shown as red spheres.
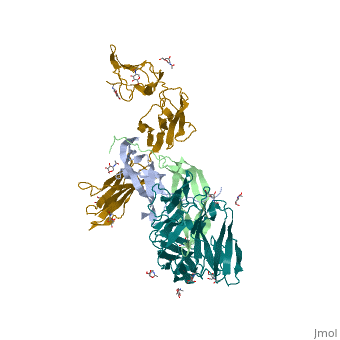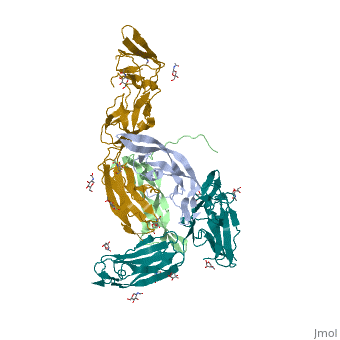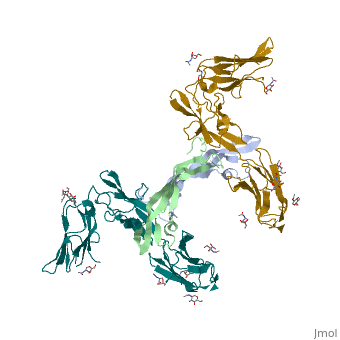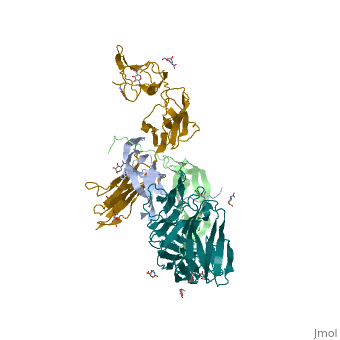
The structure of the complex between M-CSF and its receptor shows that a . There are [3]. . The via the conserved kinase DFG motif (colored in salmon) and its gatekeeper threonine residue (colored in magenta)[4]. Lapatinib is a EGFR inhibitor used in breast cancer treatment. EGFRs are overexpressed in many types of human carcinomas including lung, pancreatic, and breast cancer, and are often mutated. This overexpression leads to excessive activation of the anti-apoptotic Ras signaling cascade, resulting in uncontrolled DNA synthesis and cell proliferation. The is responsible for activating this Ras signaling cascade. Upon binding ligands like Epidermal Growth Factor, EGFR dimerizes and autophosphorylates several tyrosine residues at its C-terminal domain. Upon phosphorylation, EGFR undergoes a significant conformational shift, revealing an additional binding site capable of binding and activating downstream signaling proteins. Gefitinib inhibits the EGFR by located within the kinase domain. Residues Lys745, Leu788, Ala743, Thr790, Gln791, Met193, Pro794, Gly796, Asp800, Ser719, Glu762, & Met766 tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated. Erlotinib inhibits the EGFR by located within the kinase domain. EGFR uses residues Asp831, Lys721, Thr766, Leu820, Gly772, Phe771, Leu694, Pro770, Met769, Leu768, Gln767 & Ala719 to tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated. See also Herceptin - Mechanism of Action Ephrins (Eph) are the membrane-bound ligands of ephrin receptors. The binding of Eph and ephrin receptors is achieved via cell-cell interaction. Eph/Eph receptor signaling regulates embryonic development, guidance of axon growth, long-term potentiation, angiogenesis and stem-cell differentiation [5]. Eph-A5 is implicated in spinal cord injury. Eph-A1 is implicated in myocardial injury and renal reperfusion injury. (PDB code 3mx0).[6] . Water molecules are shown as red spheres. . The includes the N-terminal ephrin (Ligand)-binding domain (LBD), a cysteine-rich domain (CRD), and 2 fibronectin Type-III Repeats (FN3). EphA binds ephrins with . Most ephrins have a similar rigid structure which , AB, CD, FG, & GH. The LBD of EphA4 is said to be a “structural chameleon” able bind both A and B class ephrins. This explains why Ephrin Type-A receptors exhibit cross-class reactivity. The includes four important loops, the BC, DE, GH, & JK loops. EphA4 binds the GH loop of the ephrin ligand created by the EphA4 DE and JK loops. It is these loops, DE and JK, which undergo the greatest conformational shifts when binding either EphrinA2 or EphrinB2. , EphA4-Arg 162 forms a hydrogen bond with EphrinA2-Leu 138, while EphA4-Met 164 and EphA4-Leu 166 participate in hydrophobic interactions with EphrinA2-Leu 138 and EphrinA2-PHe 136. Although in the same binding pocket, the local interactions are significantly different. Most notably, the α-helix present in the EphA4-EphrinA2 JK loop is disrupted in the EphA4-EphrinB2 structure. This is due to that would occur between EphrinB2-Trp 122 and EphA4 Met 164. Instead, EphA4-Arg 162 and EphrinB2-Trp 122 form hydrophobic stacking interactions which stabilize the receptor-ligand complex. A morph of the movements EphA4 undergoes to bind EphrinA2 and EphrinB2 can be . Eph-Ephrin complexes form two unique heterotetrameric assemblies consisting of distinct EphA2-EphA2 interfaces. is generated by . The 2nd involves complex and in the region .[8] These two heterotetramers generate a (). The proximity of kinase domains in an eph-ephrin tetramer, favors transphosphorylation of tyrosines in the cytoplasmic domains. Phosphorylation promotes kinase activity by orienting the activation segment of the kinase domain in a way that favors subsrate binding and subsequent signaling. Erythropoietin (EPO) is a glycoprotein composed of only . The sulfur of the cysteine residues links to form disulfide bonds. These disulfide bonds help keep EPO's structure. Helix A is connected to Helix D by , while Helix A and Helix B are connected by . EPO’s structure was determined in 1993. It is made up of four alpha helixes. EPO is produced mainly in the kidney, but further research has shown the brain and liver still produce small amounts. The of the blood marrow is part of the hematipoietic cytokine family. This receptor has a single transmembrane domain, that forms a homodimer complex until it is activated by the binding of EPO. This receptor is 484 amino acids long and weigh 52.6 kDa. Once the homodimer is formed after the binding, autophosphorlation of the Jak2 kinases, which activates other cellular processes. This transmembrane receptor has two extracellular domains. This receptor has two disulfide bonds that are formed from 4 cystine residues, . The intracellular domain of this receptor does not possess any enzymatic activity like other receptors. When EPO comes in contact with the extracellular domains form a ligand bond. The extracellular sinding site 1 and Binding site 2 are composed of . When EPO binds, all loops on D1 and D2 of binding site one form a bind with EPO. However loop 4 of D1 on binding site 2 does not participate in the binding of EPO [9]. After the biniding of EPO, 8 tyrosine residues are phosphoralated which activates the . This kinase helps regulate the transcription of different genes and expression of other proteins. [11]. Water molecules are shown as red spheres. FGFR consist of an extracellular ligand-binding domain (LBD), transmembrane helix domain and cytoplasmic tyrosine kinase activity domain (TKD) with phosphorylated tyrosine designated PTR. FGFR LBD contains 3 immunoglobulin-like domains D1, D2 and D3. (PDB code 1evt). For more details see Group:MUZIC:Myostatin.
See also: |
| ||||||||||
ReferencesReferences
- ↑ Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem. 2014 Oct 9;57(19):7900-15. doi: 10.1021/jm501177w. Epub 2014 Sep 4. PMID:25101911 doi:http://dx.doi.org/10.1021/jm501177w
- ↑ Lee JH, Chang KZ, Patel V, Jeffery CJ. Crystal structure of rabbit phosphoglucose isomerase complexed with its substrate D-fructose 6-phosphate. Biochemistry. 2001 Jul 3;40(26):7799-805. PMID:11425306
- ↑ Felix J, De Munck S, Verstraete K, Meuris L, Callewaert N, Elegheert J, Savvides SN. Structure and Assembly Mechanism of the Signaling Complex Mediated by Human CSF-1. Structure. 2015 Jul 21. pii: S0969-2126(15)00272-5. doi:, 10.1016/j.str.2015.06.019. PMID:26235028 doi:http://dx.doi.org/10.1016/j.str.2015.06.019
- ↑ Zhang C, Ibrahim PN, Zhang J, Burton EA, Habets G, Zhang Y, Powell B, West BL, Matusow B, Tsang G, Shellooe R, Carias H, Nguyen H, Marimuthu A, Zhang KY, Oh A, Bremer R, Hurt CR, Artis DR, Wu G, Nespi M, Spevak W, Lin P, Nolop K, Hirth P, Tesch GH, Bollag G. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. Proc Natl Acad Sci U S A. 2013 Mar 14. PMID:23493555 doi:http://dx.doi.org/10.1073/pnas.1219457110
- ↑ Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007 May;17(5):230-8. Epub 2007 Apr 8. PMID:17420126 doi:http://dx.doi.org/10.1016/j.tcb.2007.03.004
- ↑ Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010 May 26. PMID:20505120
- ↑ Davis TL, Walker JR, Allali-Hassani A, Parker SA, Turk BE, Dhe-Paganon S. Structural recognition of an optimized substrate for the ephrin family of receptor tyrosine kinases. FEBS J. 2009 Aug;276(16):4395-404. PMID:19678838 doi:http://dx.doi.org/10.1111/j.1742-4658.2009.07147.x
- ↑ Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010 May 26. PMID:20505120
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Kulahin N, Kiselyov V, Kochoyan A, Kristensen O, Kastrup JS, Berezin V, Bock E, Gajhede M. Dimerization effect of sucrose octasulfate on rat FGF1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Jun 1;64(Pt, 6):448-52. Epub 2008 May 16. PMID:18540049 doi:10.1107/S174430910801066X