Growth factors: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
*[[Ephrin]] and [[Ephrin receptor]] | *[[Ephrin]] and [[Ephrin receptor]] | ||
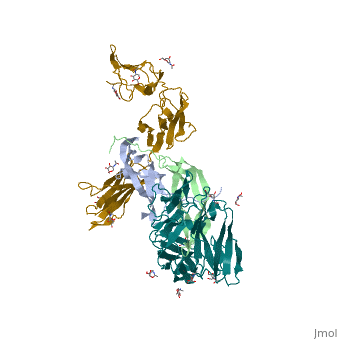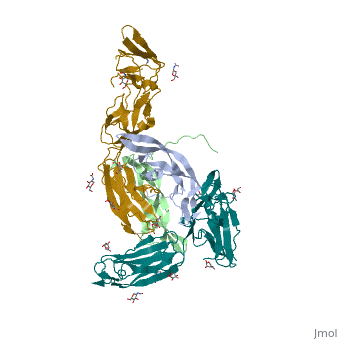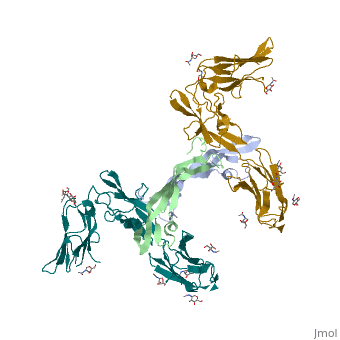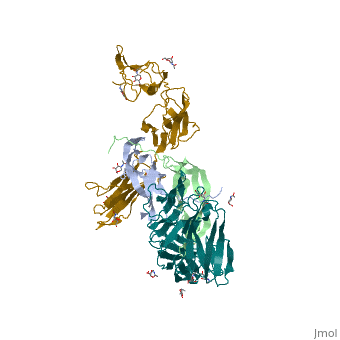
'''Ephrins''' (Eph) are the membrane-bound ligands of ephrin receptors. The binding of Eph and ephrin receptors is achieved via cell-cell interaction. Eph/Eph receptor signaling regulates embryonic development, guidance of axon growth, long-term potentiation, angiogenesis and stem-cell differentiation <ref>PMID:17420126</ref>. See also [[Ephrin receptor]]. Eph-A5 is implicated in spinal cord injury. Eph-A1 is implicated in myocardial injury and renal reperfusion injury. <scene name='59/594659/Cv/4'>Ephrin-A5 receptor-binding domain complex with ephrin type A receptor 2</scene> (PDB code [[3mx0]]).<ref>PMID:20505120</ref> | |||
*[[Erythropoietin]] and [[Erythropoietin receptor]] | *[[Erythropoietin]] and [[Erythropoietin receptor]] | ||
*[[Fibroblast growth factor]] and [[Fibroblast growth factor receptor]] | *[[Fibroblast growth factor]] and [[Fibroblast growth factor receptor]] | ||
Revision as of 14:28, 28 July 2021
3D structure of the kinase domain of Activin receptor1 (Acvr1) complex with inhibitor shows with the protein including a and a [1]. Water molecule is shown as red sphere. Rabbit [2]. Water molecules are shown as red spheres.
The structure of the complex between M-CSF and its receptor shows that a . There are [3]. . The via the conserved kinase DFG motif (colored in salmon) and its gatekeeper threonine residue (colored in magenta)[4]. Lapatinib is a EGFR inhibitor used in breast cancer treatment. EGFRs are overexpressed in many types of human carcinomas including lung, pancreatic, and breast cancer, and are often mutated. This overexpression leads to excessive activation of the anti-apoptotic Ras signaling cascade, resulting in uncontrolled DNA synthesis and cell proliferation. The is responsible for activating this Ras signaling cascade. Upon binding ligands like Epidermal Growth Factor, EGFR dimerizes and autophosphorylates several tyrosine residues at its C-terminal domain. Upon phosphorylation, EGFR undergoes a significant conformational shift, revealing an additional binding site capable of binding and activating downstream signaling proteins. Gefitinib inhibits the EGFR by located within the kinase domain. Residues Lys745, Leu788, Ala743, Thr790, Gln791, Met193, Pro794, Gly796, Asp800, Ser719, Glu762, & Met766 tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated. Erlotinib inhibits the EGFR by located within the kinase domain. EGFR uses residues Asp831, Lys721, Thr766, Leu820, Gly772, Phe771, Leu694, Pro770, Met769, Leu768, Gln767 & Ala719 to tightly bind the inhibitor. Unable to bind ATP, EGFR is incapable of autophosphorylating its C-terminal tyrosines, and the uncontrolled cell-proliferation signal is terminated. See also Herceptin - Mechanism of Action Ephrins (Eph) are the membrane-bound ligands of ephrin receptors. The binding of Eph and ephrin receptors is achieved via cell-cell interaction. Eph/Eph receptor signaling regulates embryonic development, guidance of axon growth, long-term potentiation, angiogenesis and stem-cell differentiation [5]. See also Ephrin receptor. Eph-A5 is implicated in spinal cord injury. Eph-A1 is implicated in myocardial injury and renal reperfusion injury. (PDB code 3mx0).[6]
See also: |
| ||||||||||
ReferencesReferences
- ↑ Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem. 2014 Oct 9;57(19):7900-15. doi: 10.1021/jm501177w. Epub 2014 Sep 4. PMID:25101911 doi:http://dx.doi.org/10.1021/jm501177w
- ↑ Lee JH, Chang KZ, Patel V, Jeffery CJ. Crystal structure of rabbit phosphoglucose isomerase complexed with its substrate D-fructose 6-phosphate. Biochemistry. 2001 Jul 3;40(26):7799-805. PMID:11425306
- ↑ Felix J, De Munck S, Verstraete K, Meuris L, Callewaert N, Elegheert J, Savvides SN. Structure and Assembly Mechanism of the Signaling Complex Mediated by Human CSF-1. Structure. 2015 Jul 21. pii: S0969-2126(15)00272-5. doi:, 10.1016/j.str.2015.06.019. PMID:26235028 doi:http://dx.doi.org/10.1016/j.str.2015.06.019
- ↑ Zhang C, Ibrahim PN, Zhang J, Burton EA, Habets G, Zhang Y, Powell B, West BL, Matusow B, Tsang G, Shellooe R, Carias H, Nguyen H, Marimuthu A, Zhang KY, Oh A, Bremer R, Hurt CR, Artis DR, Wu G, Nespi M, Spevak W, Lin P, Nolop K, Hirth P, Tesch GH, Bollag G. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. Proc Natl Acad Sci U S A. 2013 Mar 14. PMID:23493555 doi:http://dx.doi.org/10.1073/pnas.1219457110
- ↑ Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007 May;17(5):230-8. Epub 2007 Apr 8. PMID:17420126 doi:http://dx.doi.org/10.1016/j.tcb.2007.03.004
- ↑ Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010 May 26. PMID:20505120