MAPK/ERK pathway: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
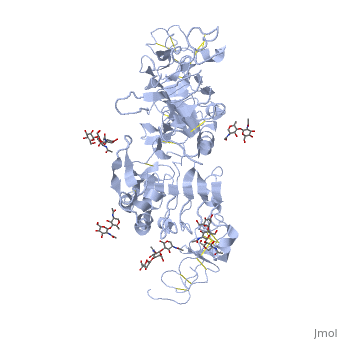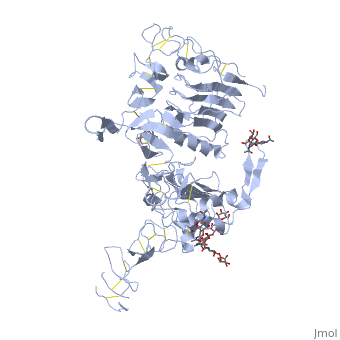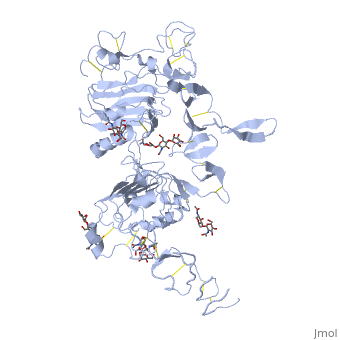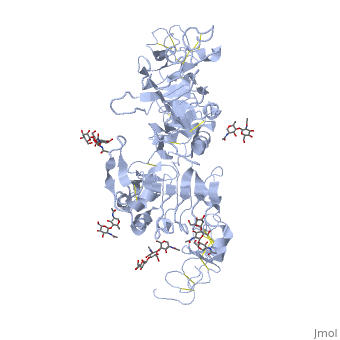
<StructureSection load='3i2t' size='350' side='right' scene='' caption='Glycosylated EGFR (PDB code [[3i2t]])'> | <StructureSection load='3i2t' size='350' side='right' scene='' caption='Glycosylated EGFR (PDB code [[3i2t]])'> | ||
MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. | MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. | ||
| Line 33: | Line 32: | ||
===MAPKs=== | ===MAPKs=== | ||
*[[Mitogen-activated protein kinase kinase]] | *[[Mitogen-activated protein kinase kinase]] '''(MEK1 or MEK2)''' | ||
*[[Mitogen-activated protein kinase]] | *[[Mitogen-activated protein kinase]] | ||
*[[Michael Roberts/BIOL115/ERK2]] | *[[Michael Roberts/BIOL115/ERK2]] | ||
| Line 47: | Line 46: | ||
====MAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. The activated CREB protein then binds to a CRE region, and is then bound to by CBP ([[CREB-binding protein]]), which coactivates it, allowing it to switch certain genes on or off. CBP is a transcription activator.==== | ====MAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. The activated CREB protein then binds to a CRE region, and is then bound to by CBP ([[CREB-binding protein]]), which coactivates it, allowing it to switch certain genes on or off. CBP is a transcription activator.==== | ||
Genes whose transcription is regulated by CREB include: ''c-fos'', BDNF, [[tyrosine hydroxylase]], numerous [[neuropeptides]] (such as | Genes whose transcription is regulated by CREB include: ''c-fos'', BDNF, [[tyrosine hydroxylase]], numerous [[neuropeptides]] (such as somatostatin, [[enkephalin]], VGF, corticotropin-releasing hormone), and genes involved in the mammalian [[circadian clock]] ([[PER1]], [[PER2]]). | ||
==Clinical significance== | |||
===Raf kinase inhibitors=== | |||
*The first drug licensed to act on this pathway is [[sorafenib]] — a Raf kinase inhibitor. | |||
*'''Dabrafenib''' ''e.g.'' [[4xv2]] - B-Raf Kinase V600E oncogenic mutant in complex with Dabrafenib. | |||
*B-Raf Kinase Inhibitor Zelboraf - Generic: '''Vemurafenib''' (Formerly: PLX-4032), see [[B-RAF with PLX4032]]. | |||
===MEK inhibitor=== | |||
*'''Cobimetinib''' or XL518, approved by US FDA in Nov 2015 for use in combination with vemurafenib (Zelboraf(R)), for treatment of advanced melanoma with a BRAF V600E or V600K mutation (see above). [[4lmn]] - MEK1 kinase bound to MEK1 kinase bound to Cobimetinib (GDC0973). | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Latest revision as of 10:00, 22 October 2023
MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. Cell surface receptors that can activate this pathway via GRB2Epidermal Growth Factor Receptor (EGFR; see also Epidermal growth factor). EGFR belongs to Receptor tyrosine kinases, class I. Trk A/B: High affinity nerve growth factor receptor (TrkA). TrkB tyrosine kinase receptor. See also Neurotrophin. Fibroblast growth factor receptor Platelet-derived growth factors and receptors Growth factor receptor-bound proteinsGrowth factor receptor-bound proteins (GRB) are adaptor proteins. GRBs contain SH2, Ras-associated (RA) and pleckstrin homology (PH) domains. For additional details on GRB10 see Grb10 SH2 Domain. Rho guanine nucleotide exchange factorRas activationAllosteric modulation of H-Ras GTPase. Kinase cascadeRAF kinaseB-Raf is related to retroviral oncogenes and participates in cellular signal transduction. B-Raf domains include the kinase domain - residues 444-721 and Ras-binding domain - residues 153-237. Mutated B-Raf was found in some human cancers[1]. See more in B-RAF with PLX4032. c-Raf is part of the MAPK pathway. c-Raf domains include the kinase domain - residues 323-618, cysteine-rich domain – residues 136-187 and Ras-binding domain - residues 51-132. Mutations of c-Raf are possible causes of Noonan syndrome[2]. For details on c-Raf see Molecular Playground/C-Raf. Mitogen-activated protein kinase kinase kinase MAPKs
Targets for phosphorylation by MAPKsOne of the first proteins known to be phosphorylated by ERK was a microtubule-associated protein (MAP)Regulation of translation and transcriptionOne effect of MAPK activation is to alter the translation of mRNA to proteins. MAPK phosphorylates the 40S ribosomal protein S6 kinase (RSK). RSK phosphorylates ribosomal protein S6.MAPK can phosphorylate C-mycMAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. The activated CREB protein then binds to a CRE region, and is then bound to by CBP (CREB-binding protein), which coactivates it, allowing it to switch certain genes on or off. CBP is a transcription activator.Genes whose transcription is regulated by CREB include: c-fos, BDNF, tyrosine hydroxylase, numerous neuropeptides (such as somatostatin, enkephalin, VGF, corticotropin-releasing hormone), and genes involved in the mammalian circadian clock (PER1, PER2). Clinical significanceRaf kinase inhibitors
MEK inhibitor
|
| ||||||||||
ReferencesReferences
- ↑ Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, Roth JA, Albelda SM, Davies H, Cox C, Brignell G, Stephens P, Futreal PA, Wooster R, Stratton MR, Weber BL. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002 Dec 1;62(23):6997-7000. PMID:12460918
- ↑ Antony R, Emery CM, Sawyer AM, Garraway LA. C-RAF mutations confer resistance to RAF inhibitors. Cancer Res. 2013 Aug 1;73(15):4840-51. doi: 10.1158/0008-5472.CAN-12-4089. Epub, 2013 Jun 4. PMID:23737487 doi:http://dx.doi.org/10.1158/0008-5472.CAN-12-4089