1gzx: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
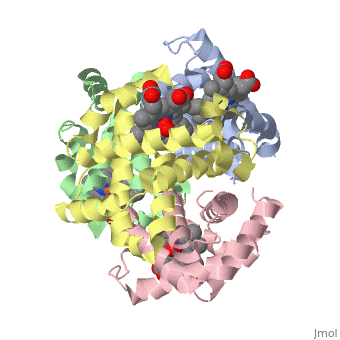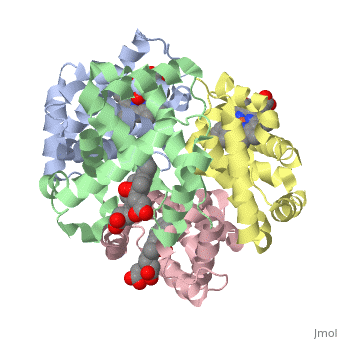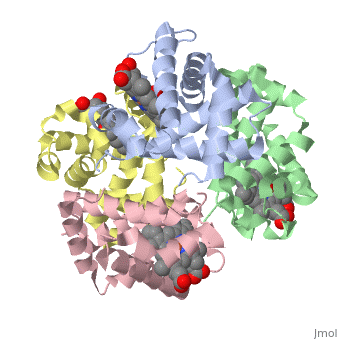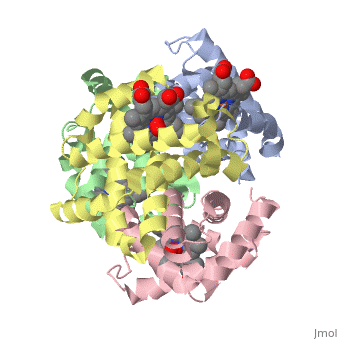
==OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS== | |||
<StructureSection load='1gzx' size='340' side='right' caption='[[1gzx]], [[Resolution|resolution]] 2.10Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1gzx]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1GZX OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1GZX FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=HEM:PROTOPORPHYRIN+IX+CONTAINING+FE'>HEM</scene>, <scene name='pdbligand=OXY:OXYGEN+MOLECULE'>OXY</scene><br> | |||
<tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1a00|1a00]], [[1a01|1a01]], [[1a0u|1a0u]], [[1a0v|1a0v]], [[1a0w|1a0w]], [[1a0x|1a0x]], [[1a0y|1a0y]], [[1a0z|1a0z]], [[1a3n|1a3n]], [[1a3o|1a3o]], [[1abw|1abw]], [[1aby|1aby]], [[1aj9|1aj9]], [[1axf|1axf]], [[1b86|1b86]], [[1bab|1bab]], [[1bbb|1bbb]], [[1bij|1bij]], [[1buw|1buw]], [[1bz0|1bz0]], [[1bz1|1bz1]], [[1bzz|1bzz]], [[1c7b|1c7b]], [[1c7c|1c7c]], [[1c7d|1c7d]], [[1cbl|1cbl]], [[1cbm|1cbm]], [[1cls|1cls]], [[1cmy|1cmy]], [[1coh|1coh]], [[1dke|1dke]], [[1dsh|1dsh]], [[1dxt|1dxt]], [[1dxu|1dxu]], [[1dxv|1dxv]], [[1g9v|1g9v]], [[1gbu|1gbu]], [[1gbv|1gbv]], [[1gli|1gli]], [[1hab|1hab]], [[1hac|1hac]], [[1hba|1hba]], [[1hbb|1hbb]], [[1hbs|1hbs]], [[1hco|1hco]], [[1hdb|1hdb]], [[1hga|1hga]], [[1hgb|1hgb]], [[1hgc|1hgc]], [[1hho|1hho]], [[1ird|1ird]], [[1j7s|1j7s]], [[1j7w|1j7w]], [[1j7y|1j7y]], [[1jy7|1jy7]], [[1k0y|1k0y]], [[1ljw|1ljw]], [[1nih|1nih]], [[1qi8|1qi8]], [[1qsh|1qsh]], [[1qsi|1qsi]], [[1rvw|1rvw]], [[1sdk|1sdk]], [[1sdl|1sdl]], [[1thb|1thb]], [[1vwt|1vwt]], [[2hbc|2hbc]], [[2hbd|2hbd]], [[2hbe|2hbe]], [[2hbf|2hbf]], [[2hbs|2hbs]], [[2hco|2hco]], [[2hhd|2hhd]], [[2hhe|2hhe]], [[4hhb|4hhb]], [[6hbw|6hbw]]</td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1gzx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1gzx OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1gzx RCSB], [http://www.ebi.ac.uk/pdbsum/1gzx PDBsum]</span></td></tr> | |||
<table> | |||
== Disease == | |||
[[http://www.uniprot.org/uniprot/HBB_HUMAN HBB_HUMAN]] Defects in HBB may be a cause of Heinz body anemias (HEIBAN) [MIM:[http://omim.org/entry/140700 140700]]. This is a form of non-spherocytic hemolytic anemia of Dacie type 1. After splenectomy, which has little benefit, basophilic inclusions called Heinz bodies are demonstrable in the erythrocytes. Before splenectomy, diffuse or punctate basophilia may be evident. Most of these cases are probably instances of hemoglobinopathy. The hemoglobin demonstrates heat lability. Heinz bodies are observed also with the Ivemark syndrome (asplenia with cardiovascular anomalies) and with glutathione peroxidase deficiency.<ref>PMID:186485</ref> <ref>PMID:6259091</ref> <ref>PMID:2599881</ref> <ref>PMID:8704193</ref> Defects in HBB are the cause of beta-thalassemia (B-THAL) [MIM:[http://omim.org/entry/613985 613985]]. A form of thalassemia. Thalassemias are common monogenic diseases occurring mostly in Mediterranean and Southeast Asian populations. The hallmark of beta-thalassemia is an imbalance in globin-chain production in the adult HbA molecule. Absence of beta chain causes beta(0)-thalassemia, while reduced amounts of detectable beta globin causes beta(+)-thalassemia. In the severe forms of beta-thalassemia, the excess alpha globin chains accumulate in the developing erythroid precursors in the marrow. Their deposition leads to a vast increase in erythroid apoptosis that in turn causes ineffective erythropoiesis and severe microcytic hypochromic anemia. Clinically, beta-thalassemia is divided into thalassemia major which is transfusion dependent, thalassemia intermedia (of intermediate severity), and thalassemia minor that is asymptomatic.<ref>PMID:1971109</ref> Defects in HBB are the cause of sickle cell anemia (SKCA) [MIM:[http://omim.org/entry/603903 603903]]; also known as sickle cell disease. Sickle cell anemia is characterized by abnormally shaped red cells resulting in chronic anemia and periodic episodes of pain, serious infections and damage to vital organs. Normal red blood cells are round and flexible and flow easily through blood vessels, but in sickle cell anemia, the abnormal hemoglobin (called Hb S) causes red blood cells to become stiff. They are C-shaped and resembles a sickle. These stiffer red blood cells can led to microvascular occlusion thus cutting off the blood supply to nearby tissues. Defects in HBB are the cause of beta-thalassemia dominant inclusion body type (B-THALIB) [MIM:[http://omim.org/entry/603902 603902]]. An autosomal dominant form of beta thalassemia characterized by moderate anemia, lifelong jaundice, cholelithiasis and splenomegaly, marked morphologic changes in the red cells, erythroid hyperplasia of the bone marrow with increased numbers of multinucleate red cell precursors, and the presence of large inclusion bodies in the normoblasts, both in the marrow and in the peripheral blood after splenectomy.<ref>PMID:1971109</ref> | |||
== Function == | |||
[[http://www.uniprot.org/uniprot/HBB_HUMAN HBB_HUMAN]] Involved in oxygen transport from the lung to the various peripheral tissues.<ref>PMID:16904236</ref> LVV-hemorphin-7 potentiates the activity of bradykinin, causing a decrease in blood pressure.<ref>PMID:16904236</ref> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/gz/1gzx_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The cooperative binding of oxygen by haemoglobin results from restraints on ligand binding in the T state. The unfavourable interactions made by the ligands at the haems destabilise the T state and favour the high affinity R state. The T <==> R equilibrium leads, in the presence of a ligand, to a rapid increase in the R state population and therefore generates cooperative binding. There is now considerable understanding of this phenomenon, but the interactions that reduce ligand affinity in the T state have not yet been fully explored, owing to the difficulties in preparing T state haemoglobin crystals in which all the subunits are oxygenated. A protocol has been developed to oxygenate deoxy T state adult human haemoglobin (HbA) crystals in air at 4 C at all four haems without significant loss of crystalline order. The X-ray crystal structure, determined to 2.1 A spacing, shows significant changes in the alpha and beta haem pockets as well as changes at the alpha(1)beta(2) interface in the direction of the R quaternary structure. Most of the shifts and deviations from deoxy T state HbA are similar to, but larger than, those previously observed in the T state met and other partially liganded T state forms. They provide clear evidence of haem-haem interaction in the T state. | |||
Crystal structure of T state haemoglobin with oxygen bound at all four haems.,Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:8642597<ref>PMID:8642597</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
==See Also== | ==See Also== | ||
*[[Ann Taylor/Hemoglobin|Ann Taylor/Hemoglobin]] | |||
*[[Hemoglobin|Hemoglobin]] | *[[Hemoglobin|Hemoglobin]] | ||
*[[Hemoglobin 3D structures|Hemoglobin 3D structures]] | |||
== | == References == | ||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Dodson, G.]] | [[Category: Dodson, G.]] | ||
Revision as of 18:48, 29 September 2014
OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMSOXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS
Structural highlights
Disease[HBB_HUMAN] Defects in HBB may be a cause of Heinz body anemias (HEIBAN) [MIM:140700]. This is a form of non-spherocytic hemolytic anemia of Dacie type 1. After splenectomy, which has little benefit, basophilic inclusions called Heinz bodies are demonstrable in the erythrocytes. Before splenectomy, diffuse or punctate basophilia may be evident. Most of these cases are probably instances of hemoglobinopathy. The hemoglobin demonstrates heat lability. Heinz bodies are observed also with the Ivemark syndrome (asplenia with cardiovascular anomalies) and with glutathione peroxidase deficiency.[1] [2] [3] [4] Defects in HBB are the cause of beta-thalassemia (B-THAL) [MIM:613985]. A form of thalassemia. Thalassemias are common monogenic diseases occurring mostly in Mediterranean and Southeast Asian populations. The hallmark of beta-thalassemia is an imbalance in globin-chain production in the adult HbA molecule. Absence of beta chain causes beta(0)-thalassemia, while reduced amounts of detectable beta globin causes beta(+)-thalassemia. In the severe forms of beta-thalassemia, the excess alpha globin chains accumulate in the developing erythroid precursors in the marrow. Their deposition leads to a vast increase in erythroid apoptosis that in turn causes ineffective erythropoiesis and severe microcytic hypochromic anemia. Clinically, beta-thalassemia is divided into thalassemia major which is transfusion dependent, thalassemia intermedia (of intermediate severity), and thalassemia minor that is asymptomatic.[5] Defects in HBB are the cause of sickle cell anemia (SKCA) [MIM:603903]; also known as sickle cell disease. Sickle cell anemia is characterized by abnormally shaped red cells resulting in chronic anemia and periodic episodes of pain, serious infections and damage to vital organs. Normal red blood cells are round and flexible and flow easily through blood vessels, but in sickle cell anemia, the abnormal hemoglobin (called Hb S) causes red blood cells to become stiff. They are C-shaped and resembles a sickle. These stiffer red blood cells can led to microvascular occlusion thus cutting off the blood supply to nearby tissues. Defects in HBB are the cause of beta-thalassemia dominant inclusion body type (B-THALIB) [MIM:603902]. An autosomal dominant form of beta thalassemia characterized by moderate anemia, lifelong jaundice, cholelithiasis and splenomegaly, marked morphologic changes in the red cells, erythroid hyperplasia of the bone marrow with increased numbers of multinucleate red cell precursors, and the presence of large inclusion bodies in the normoblasts, both in the marrow and in the peripheral blood after splenectomy.[6] Function[HBB_HUMAN] Involved in oxygen transport from the lung to the various peripheral tissues.[7] LVV-hemorphin-7 potentiates the activity of bradykinin, causing a decrease in blood pressure.[8] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe cooperative binding of oxygen by haemoglobin results from restraints on ligand binding in the T state. The unfavourable interactions made by the ligands at the haems destabilise the T state and favour the high affinity R state. The T <==> R equilibrium leads, in the presence of a ligand, to a rapid increase in the R state population and therefore generates cooperative binding. There is now considerable understanding of this phenomenon, but the interactions that reduce ligand affinity in the T state have not yet been fully explored, owing to the difficulties in preparing T state haemoglobin crystals in which all the subunits are oxygenated. A protocol has been developed to oxygenate deoxy T state adult human haemoglobin (HbA) crystals in air at 4 C at all four haems without significant loss of crystalline order. The X-ray crystal structure, determined to 2.1 A spacing, shows significant changes in the alpha and beta haem pockets as well as changes at the alpha(1)beta(2) interface in the direction of the R quaternary structure. Most of the shifts and deviations from deoxy T state HbA are similar to, but larger than, those previously observed in the T state met and other partially liganded T state forms. They provide clear evidence of haem-haem interaction in the T state. Crystal structure of T state haemoglobin with oxygen bound at all four haems.,Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:8642597[9] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||