1rgw: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
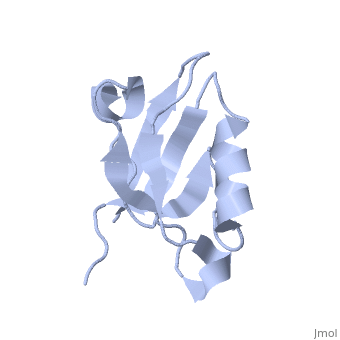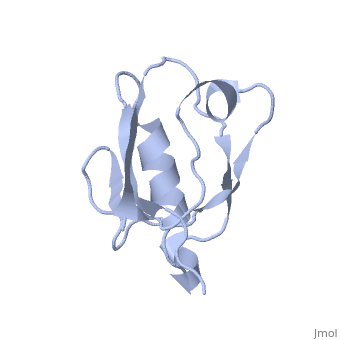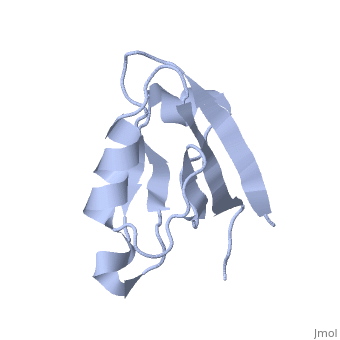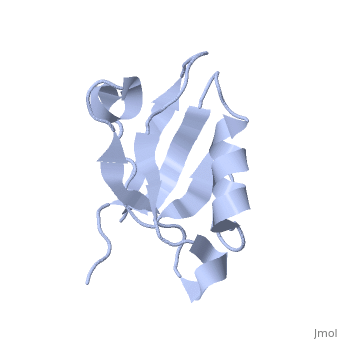
== | ==Solution Structure of ZASP's PDZ domain== | ||
[[http://www.uniprot.org/uniprot/LDB3_HUMAN LDB3_HUMAN | <StructureSection load='1rgw' size='340' side='right'caption='[[1rgw]]' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1rgw]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1RGW OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1RGW FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1rgw FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1rgw OCA], [https://pdbe.org/1rgw PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1rgw RCSB], [https://www.ebi.ac.uk/pdbsum/1rgw PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1rgw ProSAT]</span></td></tr> | |||
</table> | |||
== Disease == | |||
[https://www.uniprot.org/uniprot/LDB3_HUMAN LDB3_HUMAN] Defects in LDB3 are the cause of cardiomyopathy dilated type 1C (CMD1C) [MIM:[https://omim.org/entry/601493 601493]. Dilated cardiomyopathy is a disorder characterized by ventricular dilation and impaired systolic function, resulting in congestive heart failure and arrhythmia. Patients are at risk of premature death.<ref>PMID:14662268</ref> <ref>PMID:14660611</ref> Defects in LDB3 are the cause of left ventricular non-compaction type 3 (LVNC3) [MIM:[https://omim.org/entry/601493 601493]. Left ventricular non-compaction is characterized by numerous prominent trabeculations and deep intertrabecular recesses in hypertrophied and hypokinetic segments of the left ventricle. Defects in LDB3 are the cause of myopathy myofibrillar type 4 (MFM4) [MIM:[https://omim.org/entry/609452 609452]. A neuromuscular disorder characterized by distal and proximal muscle weakness with signs of cardiomyopathy and neuropathy. | |||
== Function == | |||
[https://www.uniprot.org/uniprot/LDB3_HUMAN LDB3_HUMAN] May function as an adapter in striated muscle to couple protein kinase C-mediated signaling via its LIM domains to the cytoskeleton.[:] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/rg/1rgw_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1rgw ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Z band alternately spliced PDZ-containing protein (ZASP) is a sarcomere Z disk protein expressed in human cardiac and skeletal muscle that is thought to be involved in a dominant familial dilated cardiomyopathy. The N-terminal PDZ domain of ZASP interacts with the C terminus of alpha-actinin-2, the major component of the Z disk, probably by forming a ternary complex with titin Z repeats. We have determined the structure of ZASP PDZ by NMR and showed that it is a classical class 1 PDZ domain that recognizes the carboxy-terminal sequence of an alpha-actinin-2 calmodulin-like domain with micromolar affinity. We also characterized the role of each component in the ternary complex ZASP/alpha-actinin-2/titin, showing that the alpha-actinin-2/ZASP PDZ interaction involves a binding surface distinct from that recognized by the titin Z repeats. ZASP PDZ structure was used to model other members of the enigma family by homology and to predict their abilities to bind alpha-actinin-2. | |||
Solution structure of ZASP PDZ domain; implications for sarcomere ultrastructure and enigma family redundancy.,Au Y, Atkinson RA, Guerrini R, Kelly G, Joseph C, Martin SR, Muskett FW, Pallavicini A, Faulkner G, Pastore A Structure. 2004 Apr;12(4):611-22. PMID:15062084<ref>PMID:15062084</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1rgw" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[ | *[[PDZ and LIM domain protein|PDZ and LIM domain protein]] | ||
*[[ZASP protein|ZASP protein]] | *[[ZASP protein|ZASP protein]] | ||
== References == | |||
== | <references/> | ||
__TOC__ | |||
</StructureSection> | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Atkinson | [[Category: Large Structures]] | ||
[[Category: Au | [[Category: Atkinson RA]] | ||
[[Category: Faulkner | [[Category: Au Y]] | ||
[[Category: Guerrini | [[Category: Faulkner G]] | ||
[[Category: Joseph | [[Category: Guerrini R]] | ||
[[Category: Martin | [[Category: Joseph C]] | ||
[[Category: Muskett | [[Category: Martin SR]] | ||
[[Category: Pallavicini | [[Category: Muskett FW]] | ||
[[Category: Pastore | [[Category: Pallavicini A]] | ||
[[Category: Pastore A]] | |||
Latest revision as of 12:04, 22 May 2024
Solution Structure of ZASP's PDZ domainSolution Structure of ZASP's PDZ domain
Structural highlights
DiseaseLDB3_HUMAN Defects in LDB3 are the cause of cardiomyopathy dilated type 1C (CMD1C) [MIM:601493. Dilated cardiomyopathy is a disorder characterized by ventricular dilation and impaired systolic function, resulting in congestive heart failure and arrhythmia. Patients are at risk of premature death.[1] [2] Defects in LDB3 are the cause of left ventricular non-compaction type 3 (LVNC3) [MIM:601493. Left ventricular non-compaction is characterized by numerous prominent trabeculations and deep intertrabecular recesses in hypertrophied and hypokinetic segments of the left ventricle. Defects in LDB3 are the cause of myopathy myofibrillar type 4 (MFM4) [MIM:609452. A neuromuscular disorder characterized by distal and proximal muscle weakness with signs of cardiomyopathy and neuropathy. FunctionLDB3_HUMAN May function as an adapter in striated muscle to couple protein kinase C-mediated signaling via its LIM domains to the cytoskeleton.[:] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedZ band alternately spliced PDZ-containing protein (ZASP) is a sarcomere Z disk protein expressed in human cardiac and skeletal muscle that is thought to be involved in a dominant familial dilated cardiomyopathy. The N-terminal PDZ domain of ZASP interacts with the C terminus of alpha-actinin-2, the major component of the Z disk, probably by forming a ternary complex with titin Z repeats. We have determined the structure of ZASP PDZ by NMR and showed that it is a classical class 1 PDZ domain that recognizes the carboxy-terminal sequence of an alpha-actinin-2 calmodulin-like domain with micromolar affinity. We also characterized the role of each component in the ternary complex ZASP/alpha-actinin-2/titin, showing that the alpha-actinin-2/ZASP PDZ interaction involves a binding surface distinct from that recognized by the titin Z repeats. ZASP PDZ structure was used to model other members of the enigma family by homology and to predict their abilities to bind alpha-actinin-2. Solution structure of ZASP PDZ domain; implications for sarcomere ultrastructure and enigma family redundancy.,Au Y, Atkinson RA, Guerrini R, Kelly G, Joseph C, Martin SR, Muskett FW, Pallavicini A, Faulkner G, Pastore A Structure. 2004 Apr;12(4):611-22. PMID:15062084[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||