1rgw
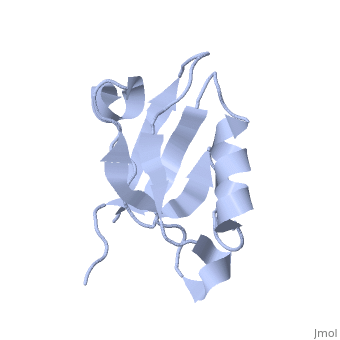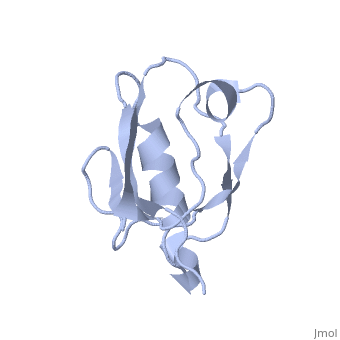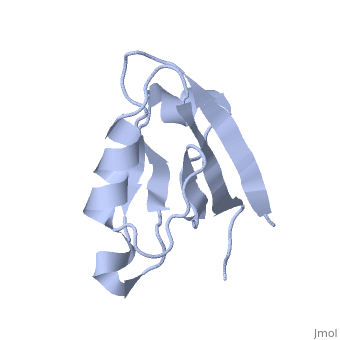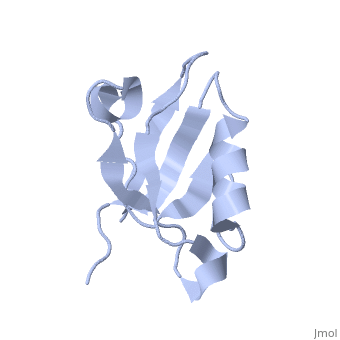
Solution Structure of ZASP's PDZ domainSolution Structure of ZASP's PDZ domain
Structural highlights
DiseaseLDB3_HUMAN Defects in LDB3 are the cause of cardiomyopathy dilated type 1C (CMD1C) [MIM:601493. Dilated cardiomyopathy is a disorder characterized by ventricular dilation and impaired systolic function, resulting in congestive heart failure and arrhythmia. Patients are at risk of premature death.[1] [2] Defects in LDB3 are the cause of left ventricular non-compaction type 3 (LVNC3) [MIM:601493. Left ventricular non-compaction is characterized by numerous prominent trabeculations and deep intertrabecular recesses in hypertrophied and hypokinetic segments of the left ventricle. Defects in LDB3 are the cause of myopathy myofibrillar type 4 (MFM4) [MIM:609452. A neuromuscular disorder characterized by distal and proximal muscle weakness with signs of cardiomyopathy and neuropathy. FunctionLDB3_HUMAN May function as an adapter in striated muscle to couple protein kinase C-mediated signaling via its LIM domains to the cytoskeleton.[:] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedZ band alternately spliced PDZ-containing protein (ZASP) is a sarcomere Z disk protein expressed in human cardiac and skeletal muscle that is thought to be involved in a dominant familial dilated cardiomyopathy. The N-terminal PDZ domain of ZASP interacts with the C terminus of alpha-actinin-2, the major component of the Z disk, probably by forming a ternary complex with titin Z repeats. We have determined the structure of ZASP PDZ by NMR and showed that it is a classical class 1 PDZ domain that recognizes the carboxy-terminal sequence of an alpha-actinin-2 calmodulin-like domain with micromolar affinity. We also characterized the role of each component in the ternary complex ZASP/alpha-actinin-2/titin, showing that the alpha-actinin-2/ZASP PDZ interaction involves a binding surface distinct from that recognized by the titin Z repeats. ZASP PDZ structure was used to model other members of the enigma family by homology and to predict their abilities to bind alpha-actinin-2. Solution structure of ZASP PDZ domain; implications for sarcomere ultrastructure and enigma family redundancy.,Au Y, Atkinson RA, Guerrini R, Kelly G, Joseph C, Martin SR, Muskett FW, Pallavicini A, Faulkner G, Pastore A Structure. 2004 Apr;12(4):611-22. PMID:15062084[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||