Role of Troponin C in decreased muscle sensitivity to calcium
IntroductionIntroduction
The article aims to discuss the molecular mechanisms of inhibition of muscular contraction by acidosis; with a specific focus on the role of the muscle regulatory protein Troponin in decreasing muscle sensitivity to calcium.
The article will be updated frequently and finalized during early May, 2013
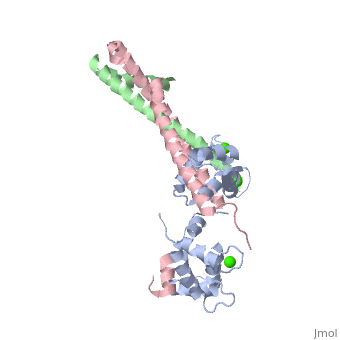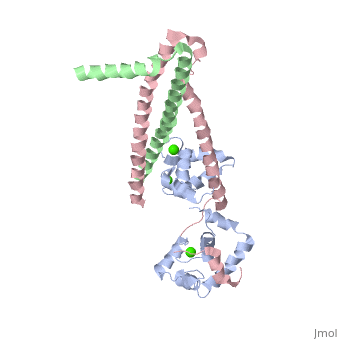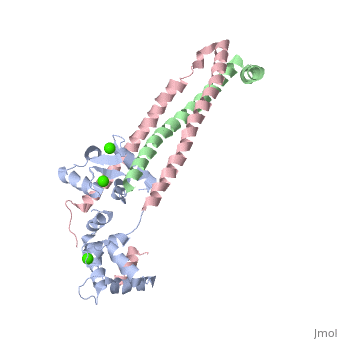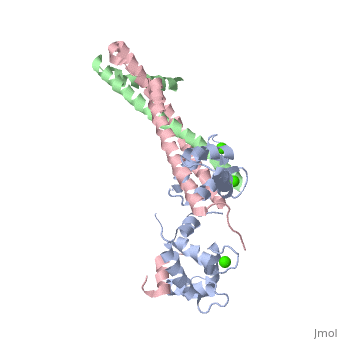
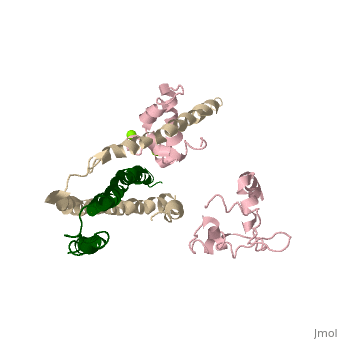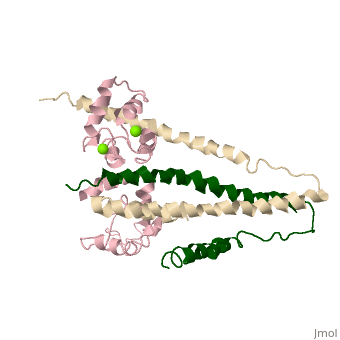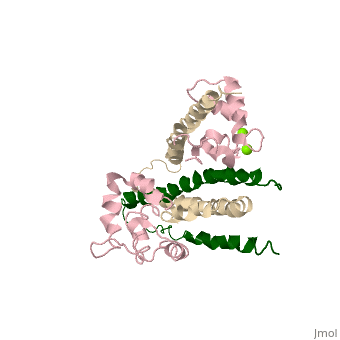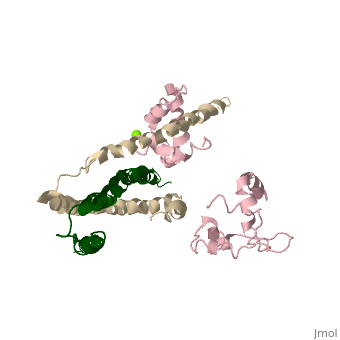
Overall Structure of Human Cardiac TroponinOverall Structure of Human Cardiac Troponin
Tn52KB shown >> Core domain = 3 subunits 1) Red = Troponin C Shown 2) Yellow = Troponin T Shown 3) Cyan = Troponin I Shown Alanine 163 is shown in red Stretch of Amphiphilic Helices shown here are in dark blue
>> Core domain also divided into two subdomains 1) Regulatory head shown
2) IT arm
>> Secondary Structure H1(I) ; H2(I) H3(I) H4(I) H1(T2) H2(T2)
Will reference Takeda et al [1]
|
| ||||||||||
Chicken Skeletal in the Ca2+ free stateChicken Skeletal in the Ca2+ free state
TroponinC Dumbbell shaped > Alpha helix linker segment connects regulatory and structural lobe. > N-terminus regulatory lobe has 2 metal binding sites (1 and 2) that prefer Ca2+. > C-terminus structural lobe has 2 metal binding sites (3 and 4) that bind Mg2+ in a relaxed muscle, and Ca2+ in active muscle.
TroponinI 104 - 115 = inhibitory segment 140 - 148 = 2nd actin binding site 116 - 131 = "Switch Segment" binds
TroponinT
|
| ||||||||||
Chicken Skeletal in the Ca2+ activated stateChicken Skeletal in the Ca2+ activated state
3 Subunits shown 1) TroponinT in yellow shown Amino acids 159-248; Maia et al found no visible electron density @249-262 Cleavage with Chymotrypsin gives two fragments: > TnT1 (1-155) Interacts with Tropomyosin (Truncated from this crystal structure) > TnT2 (156-262) required for Troponin assembly, also interacts with Tropomyosin, shown
2) TroponinI in Cyan shown Amino acids 3-143; no visible electron density for amino acids 144-182 (see section below) > Inhibitory Segment (amino acids 104-115), binds actin in the absence of Ca2+. Shown in dark blue > Second Actin binding site ( amino acids 140-148), shown in dark blue. > Switch Segment, aka transducer (amino acids 116-131) shown ; binds Calcium activated TnC's N-Terminus in exposed hydrophobic cavity. 3) TroponinC in Red shown > Four EF-Hands > Single a-helix linker connects N-Terminal regulatory (Two metal binding sites that prefer Calcium) and C-Terminal structural lobes (Two metal binding sites that can bind other metals including Mg2+ or Ca2+).
Interactions > Long helices of TnI (58-102; Cyan) and TnT2 (200-245; Yellow) = coiled coil; > (8-48) + IT coiled coil, shown = hold TnC C-Terminal domain > A-Helix linker defines dumbbell shape, approximately perpendicular to IT coil > TnC metal binding sites 1&2 (N-terminal domain) + 3&4 (C-Terminal Domain) are occupied by calcium > TnC helices B&C are in open confirmation > TnI switch segment is bound in between the EF-Hands helices. > TnI switch segment "registered" via TnC Glu-63 Gln-84 and TnI Arg-115 on pocket exterior > TnI inhibitory segment is an ordered loop that interacts with TnI via hydrophobic interactions, and interacts with TnC via electrostatic interactions only in Ca2+ activated > TnI 113-115 = unlike in cardiac uniquely ordered, lay along TnC central helix > TnI 132-143 = loop that binds N-terminal lobe of TnC > 144-182 = Disordered, and assumed to bind actin
Dumbbell shaped > Alpha helix linker segment connects regulatory and structural lobe. > N-terminus regulatory lobe has 2 metal binding sites (1 and 2) that prefer Ca2+. More precisely > C-terminus structural lobe has 2 metal binding sites (3 and 4) that bind Mg2+ in a relaxed muscle, and Ca2+ in active muscle.
104 - 115 = inhibitory segment 140 - 148 = 2nd actin binding site 116 - 131 = "Switch Segment" binds
|
| ||||||||||
CRYSTAL STRUCTURE OF CALCIUM-SATURATED RABBIT SKELETAL TROPONIN CCRYSTAL STRUCTURE OF CALCIUM-SATURATED RABBIT SKELETAL TROPONIN C
Shown as a ribbon diagram, metal ions are shown in black. Contains the following helices N helix (1-10) A helix (13-26)
(36-46) within this helix lies a as a ball and stick model in yellow The same is shown in orientation. Helix B and linker moves away from N/A, 35-57 moves away from 70-83. Finally B/C moves with respect to A/D causing an open conformation. C helix (52-62) D helix (72-83) Contains the following helices E helix (93-102) F helix (112-122) G helix (128-138) H helix (148-156)
|
| ||||||||||
Rabbit Skeletal Troponin C in complex with a short Troponin I fragmentRabbit Skeletal Troponin C in complex with a short Troponin I fragment
TnC is shown in red; the N-terminus is to the right of the window as oriented. The short 47 residue fragment of TnI is transparent green. The B-Helix of TnC is shown V43 is shown in yellow as a ball and stick model. The N-terminal domain with V43 as a ball and stick in a rear view perspective is shown
|
| ||||||||||
Actin binding domain of chicken Troponin IActin binding domain of chicken Troponin I
Murakami K, Yumoto F, Ohki SY, Yasunaga T, Tanokura M, et al. (2005) Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol 352: 178–201 Key phrase in the abstract is "The mobile domain appears to tumble independently of the core domain of troponin."
155-182 in dark blue R155 in red
|
| ||||||||||