1j1e
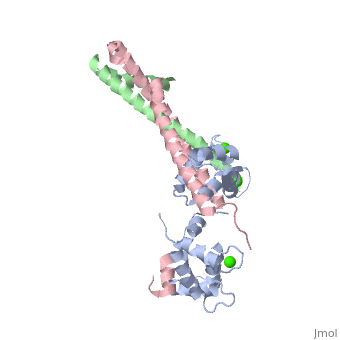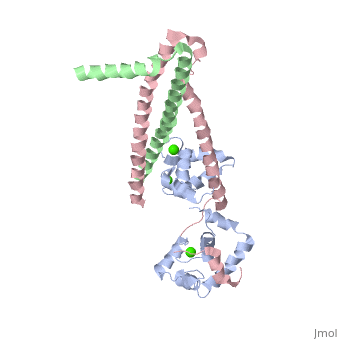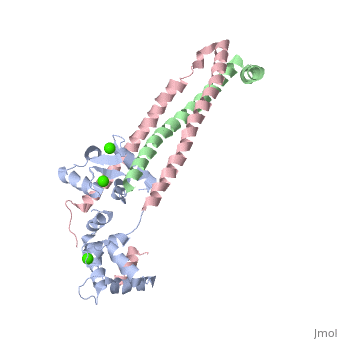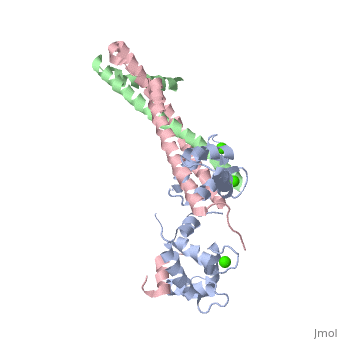
Crystal structure of the 52kDa domain of human cardiac troponin in the Ca2+ saturated formCrystal structure of the 52kDa domain of human cardiac troponin in the Ca2+ saturated form
Structural highlights
DiseaseTNNC1_HUMAN Defects in TNNC1 are the cause of cardiomyopathy dilated type 1Z (CMD1Z) [MIM:611879. Dilated cardiomyopathy is a disorder characterized by ventricular dilation and impaired systolic function, resulting in congestive heart failure and arrhythmia. Patients are at risk of premature death.[1] Defects in TNNC1 are the cause of familial hypertrophic cardiomyopathy type 13 (CMH13) [MIM:613243. A hereditary heart disorder characterized by ventricular hypertrophy, which is usually asymmetric and often involves the interventricular septum. The symptoms include dyspnea, syncope, collapse, palpitations, and chest pain. They can be readily provoked by exercise. The disorder has inter- and intrafamilial variability ranging from benign to malignant forms with high risk of cardiac failure and sudden cardiac death.[2] [3] [4] [5] FunctionTNNC1_HUMAN Troponin is the central regulatory protein of striated muscle contraction. Tn consists of three components: Tn-I which is the inhibitor of actomyosin ATPase, Tn-T which contains the binding site for tropomyosin and Tn-C. The binding of calcium to Tn-C abolishes the inhibitory action of Tn on actin filaments. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedTroponin is essential in Ca(2+) regulation of skeletal and cardiac muscle contraction. It consists of three subunits (TnT, TnC and TnI) and, together with tropomyosin, is located on the actin filament. Here we present crystal structures of the core domains (relative molecular mass of 46,000 and 52,000) of human cardiac troponin in the Ca(2+)-saturated form. Analysis of the four-molecule structures reveals that the core domain is further divided into structurally distinct subdomains that are connected by flexible linkers, making the entire molecule highly flexible. The alpha-helical coiled-coil formed between TnT and TnI is integrated in a rigid and asymmetric structure (about 80 angstrom long), the IT arm, which bridges putative tropomyosin-anchoring regions. The structures of the troponin ternary complex imply that Ca(2+) binding to the regulatory site of TnC removes the carboxy-terminal portion of TnI from actin, thereby altering the mobility and/or flexibility of troponin and tropomyosin on the actin filament. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form.,Takeda S, Yamashita A, Maeda K, Maeda Y Nature. 2003 Jul 3;424(6944):35-41. PMID:12840750[6] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||