Ricin
This page, as it appeared on May 14, 2013, was featured in this article in the journal Biochemistry and Molecular Biology Education.
Ricin is a potent cytotoxin that is synthesized in the endosperm cells of maturing seeds of the castor oil plant (Ricinus communis)[1]. Ricin belongs to a small multi-gene family[2] that is composed of eight members. Ricin is classified as a type II heterodimeric Ribosome Inactivating Protein[1] or RIPs. For toxins in Proteopedia see Toxins.
See also Ricin: A toxic protein; Ricin: Structure and function.
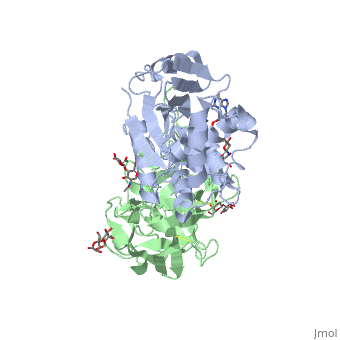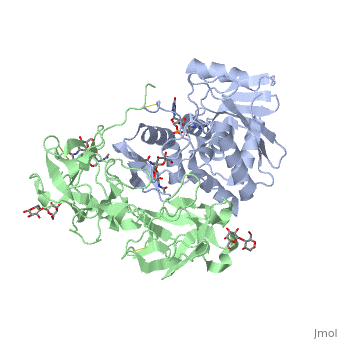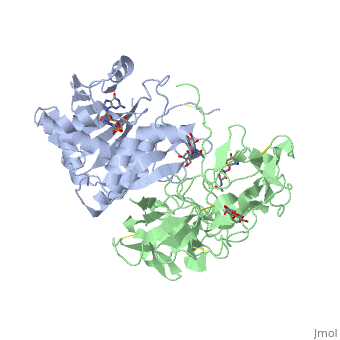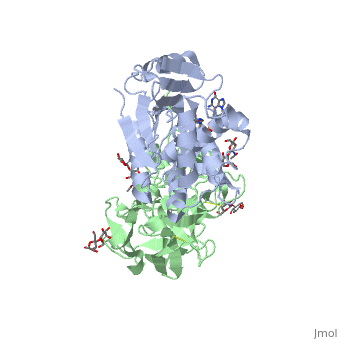
StructureRicin is a heterodimer that consists of a 32 kilodalton A chain glycoprotein (light blue) linked by a to a 32 kilodalton glycoprotein[2] (green). The is an alpha/beta protein which contains eight alpha helices (pink) and eight beta sheets (yellow). It has three domains[3]. consists of a beta sheet containing both parallel and anti-parallel strands. The makes up the core of the protein, and includes the active site. The interacts with the B chain, and contains a helix and two beta strands. The A chain contains the active site that is responsible for inactivating the Ribosome via depurination. RIPs have very diverse structures, containing only eight invariant residues[1]. These are clustered in the active site. The B chain is a lectin[1] that to galactose-containing surface receptors. Originally it was thought that the mode of action of Ricin poisoning was due to hemagglutination due to a closely related, co-isolating lectin, RCA. Mechanism of actionThe mechanism deployed by Ricin to gain entry to a host cell involves the poison's heterogenic properties. First, the B subunit binds to two carbohydrates on the cell surface, either glycolipids or glycoproteins, which both terminate with galactose. The interaction is facilitated by hydrogen bonds to in one domain[4] and in the other domain. Once bound, the ricin-glycoprotein complex is taken into the cells via endocytosis. This association between the A and B chain is essential for toxicity [2] without it the Ricin would not be able to gain access to the cell, rendering it useless[5]. The endocytotic pathway results in the cleavage of the disulfide bond linking the A and B chains. After cleavage, the A chain is released into the cytosol. Once the A chain gains into the cytosol, it depurinates a single adenosine residue in a highly conserved portion within the large ribosomal subunit[5] of eukaryotes; in human, the large cytoplasmic ribosomal RNA is called the 28S ribosomal RNA because of its sedimentation properties during ultracentrifugation. The nucleotide depurinated is located within a specific, conserved loop referred to as the 'sarcin-ricin loop'. Depurination of the single adenosine nucleotide by the toxin results in the inhibition of protein synthesis. The proposed mechanism of depurination utilizes the in the A chain. The aromatic ring structures of the substrate adenosine stack with the aromatic side chains of , Tyr 80 and 123, above and below. Hydrogen bonds form between the conserved arginine and a backbone carbonyl. The depurination reaction is aided by the protonation of N3 by Arg 180 and by ion pairing to Glu 177. A water molecule on the opposite side of the ribose is activated by hydrogen bonding to Arg 180. The activated water attacks C1' of the ribose, releasing the adenine and depurinated RNA fragment. This interferes with elongation factor binding to the ribosome, thus inhibiting translation. Site of ricin modification of rRNARicin removes an adenine from a specific portion of the 28S rRNA called the , or SRL. This leads to reduced binding of elongation factors to the ribosome and reduced synthesis of proteins[6]. It appears that binding of ricin chain A to the ribosome is mediated by binding to the ribosomal proteins and the ribosomal stalk, as binding to the naked rRNA occurs with lower affinity.[7]. Ricin also triggers apoptosis [8], though the exact pathway is a current research topic. There is some evidence that it occurs via the B subunit [9], though there is also evidence that the protein synthesis inhibition may cause apoptosis [10]. |
| ||||||||||
3D structures of ricin3D structures of ricin
See AlsoSee Also
ReferencesReferences
- ↑ 1.0 1.1 1.2 1.3 Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID:8119491
- ↑ 2.0 2.1 2.2 Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, Rutenber E, Xuong NH, Hamlin R, Robertus JD. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987 Apr 15;262(11):5398-403. PMID:3558397
- ↑ Weston SA, Tucker AD, Thatcher DR, Derbyshire DJ, Pauptit RA. X-ray structure of recombinant ricin A-chain at 1.8 A resolution. J Mol Biol. 1994 Dec 9;244(4):410-22. PMID:7990130 doi:http://dx.doi.org/10.1006/jmbi.1994.1739
- ↑ Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987 Apr 9-15;326(6113):624-6. PMID:3561502 doi:http://dx.doi.org/10.1038/326624a0
- ↑ 5.0 5.1 Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3783-8. PMID:9108055
- ↑ Holmberg L, Nygard O. Depurination of A4256 in 28 S rRNA by the ribosome-inactivating proteins from barley and ricin results in different ribosome conformations. J Mol Biol. 1996 May 31;259(1):81-94. PMID:8648651 doi:10.1006/jmbi.1996.0303
- ↑ Chiou JC, Li XP, Remacha M, Ballesta JP, Tumer NE. The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin A chain in Saccharomyces cerevisiae. Mol Microbiol. 2008 Dec;70(6):1441-52. doi: 10.1111/j.1365-2958.2008.06492.x., Epub 2008 Oct 30. PMID:19019145 doi:10.1111/j.1365-2958.2008.06492.x
- ↑ Tesh VL. The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol. 2012;357:137-78. doi: 10.1007/82_2011_155. PMID:22130961 doi:10.1007/82_2011_155
- ↑ Yermakova A, Vance DJ, Mantis NJ. Sub-domains of ricin's B subunit as targets of toxin neutralizing and non-neutralizing monoclonal antibodies. PLoS One. 2012;7(9):e44317. doi: 10.1371/journal.pone.0044317. Epub 2012 Sep 11. PMID:22984492 doi:10.1371/journal.pone.0044317
- ↑ Jetzt AE, Cheng JS, Li XP, Tumer NE, Cohick WS. A relatively low level of ribosome depurination by mutant forms of ricin toxin A chain can trigger protein synthesis inhibition, cell signaling and apoptosis in mammalian cells. Int J Biochem Cell Biol. 2012 Dec;44(12):2204-11. doi:, 10.1016/j.biocel.2012.09.004. Epub 2012 Sep 12. PMID:22982239 doi:10.1016/j.biocel.2012.09.004