3rtj
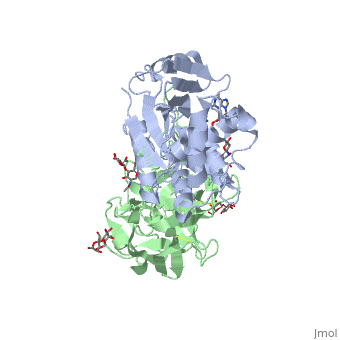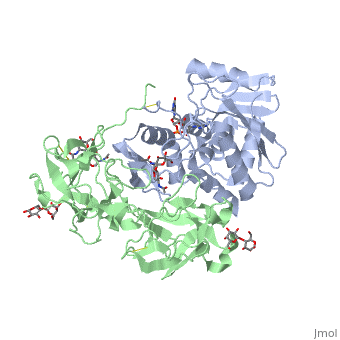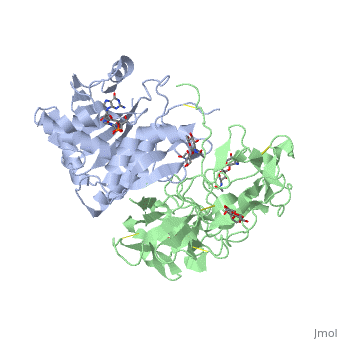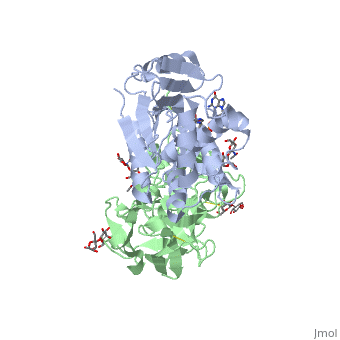
Crystal structure of ricin bound with dinucleotide ApGCrystal structure of ricin bound with dinucleotide ApG
Structural highlights
Function[RICI_RICCO] Ricin is highly toxic to animal cells and to a lesser extent to plant cells. The A chain acts as a glycosidase that removes a specific adenine residue from an exposed loop of the 28S rRNA (A4324 in mammals), leading to rRNA breakage. As this loop is involved in elongation factor binding, modified ribosomes are catalytically inactive and unable to support protein synthesis. The A chain can inactivate a few thousand ribosomes per minute, faster than the cell can make new ones. Therefore a single A chain molecule can kill an animal cell. The B chain binds to beta-D-galactopyranoside moieties on cell surface glycoproteins and glycolipids and facilitates the entry into the cell of the A chain; B chains are also responsible for cell agglutination (Lectin activity). Publication Abstract from PubMedRicin A-chain is an N-glycosidase that hydrolyzes the adenine ring from a specific adenosine of rRNA. Formycin monophosphate (FMP) and adenyl(3'-->5')guanosine (ApG) were bound to ricin A-chain and their structures elucidated by X-ray crystallography. The formycin ring stacks between tyrosines 80 and 123 and at least four hydrogen bonds are made to the adenine moiety. A residue invariant in this enzyme class, Arg180, appears to hydrogen bond to N-3 of the susceptible adenine. Three hypothetical models for binding a true hexanucleotide substrate, CGAGAG, are proposed. They incorporate adenine binding, shown by crystallography, but also include geometry likely to favor catalysis. For example, efforts have been made to orient the ribose ring in a way that allows solvent attack and oxycarbonium stabilization by the enzyme. The favored model is a simple perturbation of the tetraloop structure determined by nuclear magnetic resonance for similar polynucleotides. The model is attractive in that specific roles are defined for conserved protein residues. A mechanism of action is proposed. It invokes oxycarbonium ion stabilization on ribose by Glu177 in the transition state. Arg180 stabilizes anion development on the leaving adenine by protonation at N-3 and may activate a trapped water molecule that is the ultimate nucleophile in the depurination. X-ray analysis of substrate analogs in the ricin A-chain active site.,Monzingo AF, Robertus JD J Mol Biol. 1992 Oct 20;227(4):1136-45. PMID:1433290[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||||