4iar
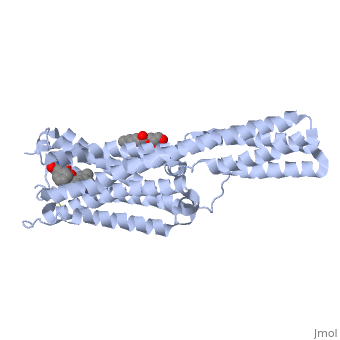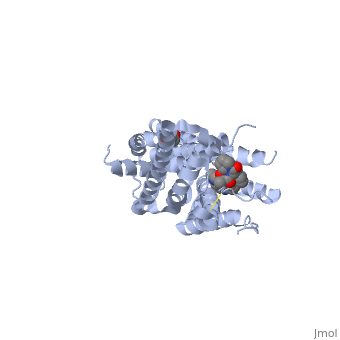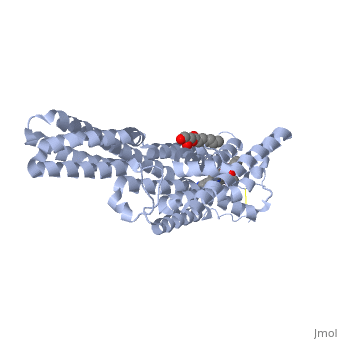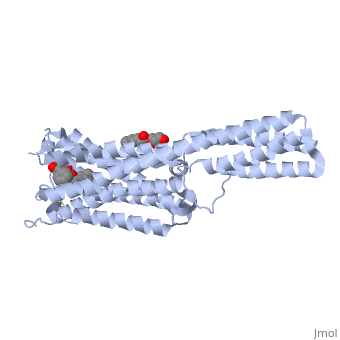
Crystal structure of the chimeric protein of 5-HT1B-BRIL in complex with ergotamine (PSI Community Target)Crystal structure of the chimeric protein of 5-HT1B-BRIL in complex with ergotamine (PSI Community Target)
Structural highlights
FunctionC562_ECOLX Electron-transport protein of unknown function.5HT1B_HUMAN This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that inhibit adenylate cyclase activity. Publication Abstract from PubMedSerotonin or 5-hydroxytryptamine (5-HT) regulates a wide spectrum of human physiology through the 5-HT receptor family. We report the crystal structures of the human 5-HT1B G protein-coupled receptor bound to the agonist antimigraine medications ergotamine and dihydroergotamine. The structures reveal similar binding modes for these ligands, which occupy the orthosteric pocket and an extended binding pocket close to the extracellular loops. The orthosteric pocket is formed by residues conserved in the 5-HT receptor family, clarifying the family-wide agonist activity of 5-HT. Compared with the accompanying structure of the 5-HT2B receptor, the 5-HT1B receptor displays a 3-angstrom outward shift at the extracellular end of helix V, resulting in a more open extended pocket that explains subtype selectivity. Together with docking and mutagenesis studies, these structures provide a comprehensive structural basis for understanding receptor-ligand interactions and designing subtype-selective serotonergic drugs. Structural Basis for Molecular Recognition at Serotonin Receptors.,Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W, Huang XP, Vardy E, McCorvy JD, Gao X, Zhou EX, Melcher K, Zhang C, Bai F, Yang H, Yang L, Jiang H, Roth BL, Cherezov V, Stevens RC, Xu HE Science. 2013 Mar 21. PMID:23519210[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||