2x0c
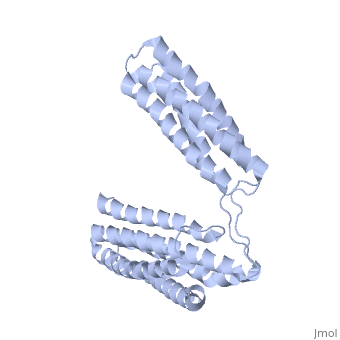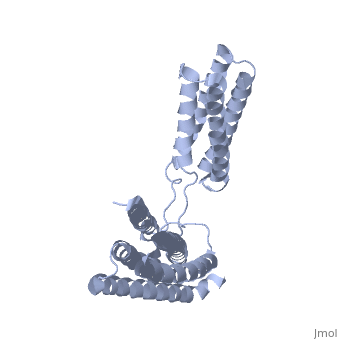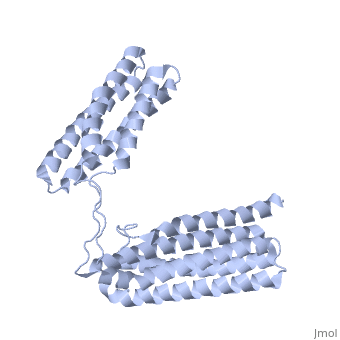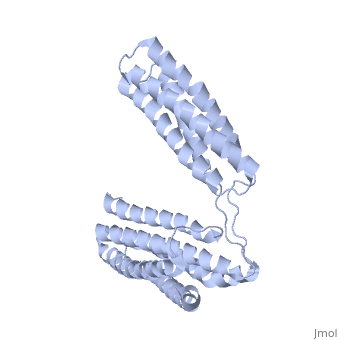
STRUCTURE OF THE TALIN ROD RESIDUES 1359-1659STRUCTURE OF THE TALIN ROD RESIDUES 1359-1659
Structural highlights
Function[TLN1_MOUSE] Probably involved in connections of major cytoskeletal structures to the plasma membrane. High molecular weight cytoskeletal protein concentrated at regions of cell-substratum contact and, in lymphocytes, at cell-cell contacts. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedTalin is an adaptor protein that couples integrins to F-actin. Structural studies show that the N-terminal talin head contains an atypical FERM domain while the N- and C-terminal parts of the talin rod comprise a series of alpha-helical bundles. However, determining the structure of the central part of the rod has proved problematic. Residues 1359-1659 are homologous to the MESDc1 gene product, and we therefore expressed this region of talin in E. coli. The crystal structure shows a unique fold comprised of a 5- and 4-helix bundle. The 5-helix bundle is composed of non-sequential helices due to insertion of the 4-helix bundle into the loop at the C-terminus of helix alpha3. The linker connecting the bundles forms a two-stranded anti-parallel beta-sheet likely limiting the relative movement of the two bundles. Because the 5-helix bundle contains the N- and C-termini of this module, we propose that it is linked by short loops to adjacent bundles while the 4-helix bundle protrudes from the rod. This suggests the 4-helix bundle has a unique role, and its pI (7.8) is higher than other rod domains. Both helical bundles contain vinculin-binding sites, but that in the isolated 5-helix bundle is cryptic whereas that in the isolated 4-helix bundle is constitutively active. In contrast, both bundles are required for actin binding. Finally, we show that the MESDc1 protein, which is predicted to have a similar fold, is a novel actin binding protein. The central region of talin has a unique fold that binds vinculin and actin.,Gingras AR, Bate N, Goult BT, Patel B, Kopp PM, Emsley J, Barsukov IL, Roberts GC, Critchley DR J Biol Chem. 2010 Jul 7. PMID:20610383[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||