1jun
NMR STUDY OF C-JUN HOMODIMER
|
OverviewOverview
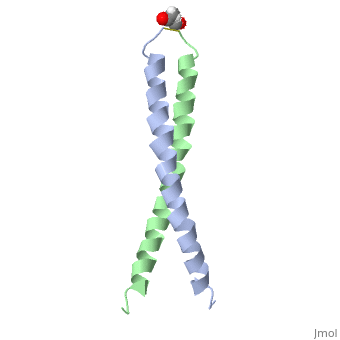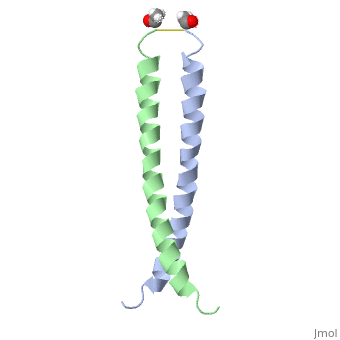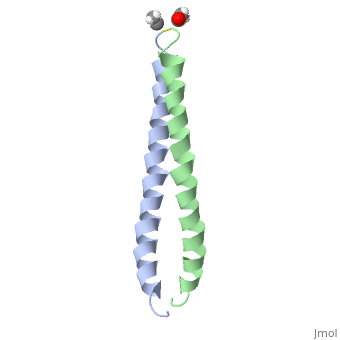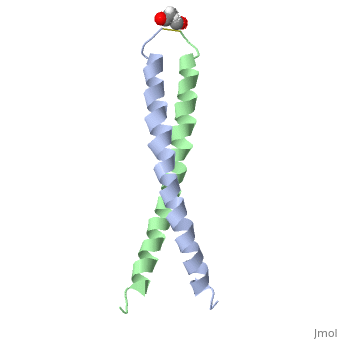
The solution structure of the c-Jun leucine zipper domain has been determined to high resolution using a new calculation protocol designed to handle highly ambiguous sets of interproton distance restraints. The domain comprises a coiled coil of parallel alpha-helices in which most of the hydrophobic residues are buried at the highly symmetrical dimer interface; this interface extends over 10 helical turns and is the most elongated protein domain solved to date using NMR methods. The backbone fold is very similar to that seen in crystal structures of the GCN4 and Jun-Fos leucine zippers; however, in contrast with these crystal structures, the Jun leucine zipper dimer appears to be devoid of favorable intermolecular electrostatic interactions. A polar asparagine residue, located at the dimer interface, forms the sole point of asymmetry in the structure; furthermore, the side chain of this residue is disordered due to motional averaging. This residue, which is highly conserved in the leucine zipper family of transcription factors, provides a destabilizing influence that is likely to facilitate the rapid exchange of zipper strands in vivo.
DiseaseDisease
Known diseases associated with this structure: Autoimmune polyglandular disease, type I OMIM:[607358], Sveinsson choreoretinal atrophy OMIM:[189967]
About this StructureAbout this Structure
1JUN is a Single protein structure of sequence from Homo sapiens with as ligand. Full crystallographic information is available from OCA.
ReferenceReference
High resolution NMR solution structure of the leucine zipper domain of the c-Jun homodimer., Junius FK, O'Donoghue SI, Nilges M, Weiss AS, King GF, J Biol Chem. 1996 Jun 7;271(23):13663-7. PMID:8662824
Page seeded by OCA on Thu Feb 21 13:26:57 2008