1jun
NMR STUDY OF C-JUN HOMODIMERNMR STUDY OF C-JUN HOMODIMER
Structural highlights
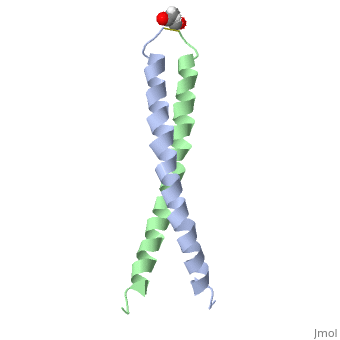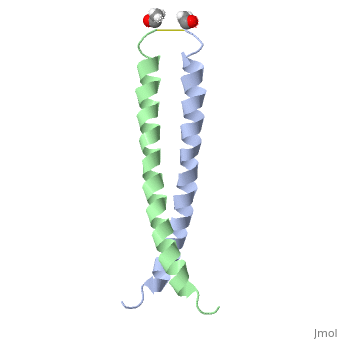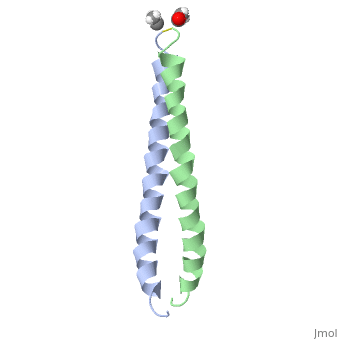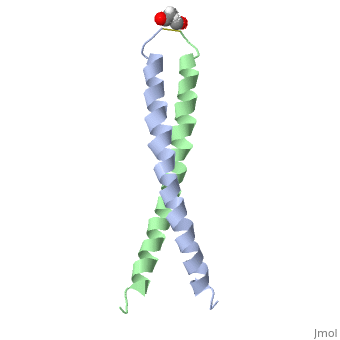
FunctionJUN_HUMAN Transcription factor that recognizes and binds to the enhancer heptamer motif 5'-TGA[CG]TCA-3'. Promotes activity of NR5A1 when phosphorylated by HIPK3 leading to increased steroidogenic gene expression upon cAMP signaling pathway stimulation.[1] [2] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe solution structure of the c-Jun leucine zipper domain has been determined to high resolution using a new calculation protocol designed to handle highly ambiguous sets of interproton distance restraints. The domain comprises a coiled coil of parallel alpha-helices in which most of the hydrophobic residues are buried at the highly symmetrical dimer interface; this interface extends over 10 helical turns and is the most elongated protein domain solved to date using NMR methods. The backbone fold is very similar to that seen in crystal structures of the GCN4 and Jun-Fos leucine zippers; however, in contrast with these crystal structures, the Jun leucine zipper dimer appears to be devoid of favorable intermolecular electrostatic interactions. A polar asparagine residue, located at the dimer interface, forms the sole point of asymmetry in the structure; furthermore, the side chain of this residue is disordered due to motional averaging. This residue, which is highly conserved in the leucine zipper family of transcription factors, provides a destabilizing influence that is likely to facilitate the rapid exchange of zipper strands in vivo. High resolution NMR solution structure of the leucine zipper domain of the c-Jun homodimer.,Junius FK, O'Donoghue SI, Nilges M, Weiss AS, King GF J Biol Chem. 1996 Jun 7;271(23):13663-7. PMID:8662824[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||