Tom Sandbox

| |||||||
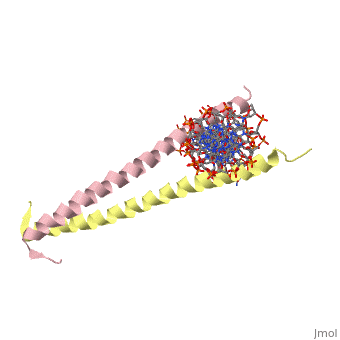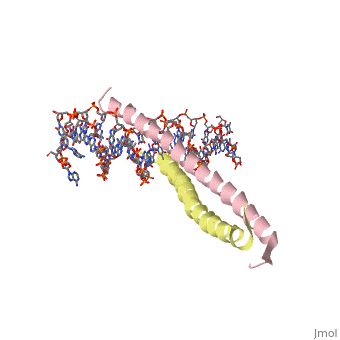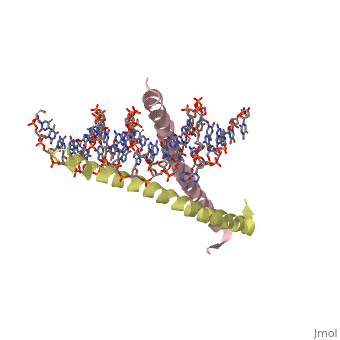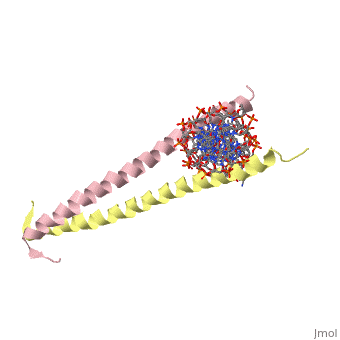
| 2zta, resolution 1.80Å () | |||||||
|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
GCN4 - The Leucine ZipperGCN4 - The Leucine Zipper
GCN4 (PDB 2zta by itself, 1ysa bound to DNA) is a eukaryotic transcription factor first isolated from Saccharomyces cerevisiae, also known as Baker's Yeast. The first 'leucine zipper' model was coined by Landshulz et al. in 1988. Today, while the name has stayed the same, we no longer view the leucine binding region in an inter-collated manner, but as leucines meeting face to face [1]. (see heptad repeat section)
StructureStructure
GCN4 is composed of two identical 58 residue alpha helix chains that grouped together to form a parallel coiled-coil dimer. The dimer binds through interlocking leucine amino acids and hydrophobic residues near the C terminus, while pinching in on the major groove of DNA in the N terminal end via basic residues. These two main domains are thus labled the leucine zipper dimerization domain and the basic DNA-binding domain. [2] The basic residues are the reason the class of binding interactions is commonly referred to as bZIP or basic region leucine zipper proteins[3]. The X-ray structure of the 33-residue polypeptide corresponding to the leucine zipper of GCN4 was determined by Peter Kim and Thomas Alber[4]
Heptad RepeatHeptad Repeat
GCN4 is said to have a heptad repeat, or seven unit repeated sequence. Here the heptad repeat is a leucine, every seven units. When the structure of the alpha helices is taken into account, it is clear that each leucine is being stacked on top of the last one, every seven units. This creates the zipper-like repeated projections from the two polypeptide chains. Below we see a pictorial representation of the sequence of amino acids as we look down the length of the coiled coil structure.
| |||||||||
| 1ysa, resolution 2.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Image 1:A pictorial representation of the heptad repeat between the two subunits in a coiled-coil conformation. By following through the wheels alphabetically, it is clear how the leucines will stack every 7 units. The apostrophe denotes the diference between the two polypeptide subunits.(from Kgutwin in Wikipedia Commons http://commons.wikimedia.org/wiki/File:Coiledcoil-wheelcartoon.gif)
At every d and d' we have a Leucine, while at the a and a' locations we typically see valine. [3] In the opposite positions one tends to see more charged and polar residues. The Leucine and Valine repeats create a strongly hydrophobic region between the two alpha helices. This clearly shows the relationship between coils and shown below, we can see that for the two helices to be symmetrical, the Leucines must not in fact be oriented as a zipper, but on the same levels.
The Leucine ZipperThe Leucine Zipper
The leucines themselves come on every other level of the alpha helix and do not actually interchange one over the other like a zipper, but instead make side to side contact. This was noted in 1990 due to the symmetric nature of the two subunits. If the leucines showed interdigitation the subunits would be asymmetric [1].
The Leucine Zipper of GCN4, as expected, operates under a specific pH. It has been shown that at lower pH values the zipper will reversibly protenate and open up, losing its hydrophobic stability. Researchers are looking into this opening and closing reaction as a form of nano-tweezers. [2]
Image 2: A schematic representation of the Leucine Zipper acting as nano-tweezers under different pH conditions[2].
Binding with DNABinding with DNA
The basic region binding domain of GCN4 causes a shift in bothe the AP-1 and ATF/CREB binding sites of DNA[5].
FunctionFunction
See AlsoSee Also
ReferenceReference
- ↑ 1.0 1.1 Oas, T. G.; McIntosh, L. P.; O'Shea, E. K.; Dahlquist, F. W.; and Kim, P. S. Biochemistry 1990 29 (12), 2891-2894
- ↑ 2.0 2.1 2.2 Sharma, G.; Rege, K.; Budil, D. E.; Yarmush, M. L.; Mavroidis, C. Int J Nanomedicine. 2008 December; 3(4): 505–521.
- ↑ 3.0 3.1 Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008.
- ↑ O'Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991 Oct 25;254(5031):539-44. PMID:1948029
- ↑ Dragan AI, Liu Y, Makeyeva EN, Privalov PL. DNA-binding domain of GCN4 induces bending of both the ATF/CREB and AP-1 binding sites of DNA. Nucleic Acids Res. 2004 Sep 30;32(17):5192-7. Print 2004. PMID:15459288 doi:10.1093/nar/gkh854