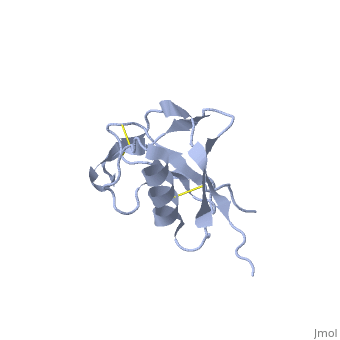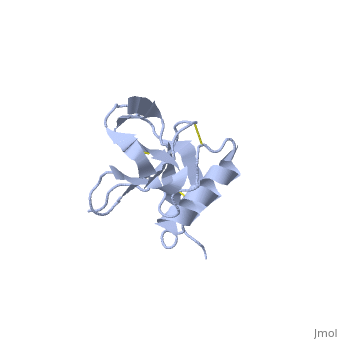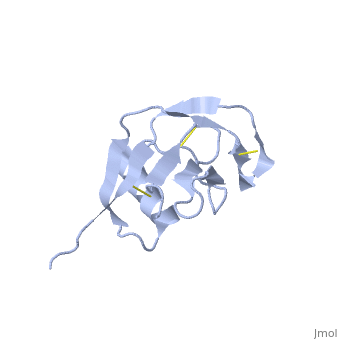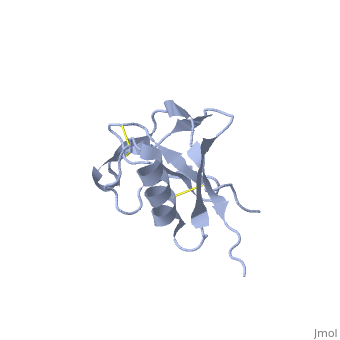
Amyloid precursor protein (APP) is thought to regulate transcription. APP is cleaved by β-secretase and the resulting N terminal peptide which is ca. 40 amino acid long is called hAPP β-peptide. The aggregation of the hAPP β-peptide is the cause of Alzheimer’s Disease. For detailed discussion see
3D structures of amyloid precursor protein3D structures of amyloid precursor protein
Updated on 17-November-2014
{"openlevels":0}
- APP
- hAPP β-peptide
- 1z0q, 2beg – hAPP β-peptide residues 1-42 – NMR
- 1amb, 1amc – hAPP β-peptide residues 1-28 – NMR
- 1qcm, 1qwp, 1qxc, 1qyt – hAPP β-peptide residues 25-35 – NMR
- 1ba4, 1ba6, 2lnq – hAPP β-peptide residues 1-40 – NMR
- 1bjb, 1bjc – hAPP β-peptide residues 1-28 (mutant) – NMR
- 1nmj – APP β-peptide residues 1-28 – rat – NMR
- 3bae – hAPP β-peptide residues 1-28 + antibody
- 3bkj – hAPP β-peptide residues 1-16 + antibody
- 2roz – hAPP β-peptide residues 1-32 + amyloid β A4 precursor protein - NMR
- 2wk3 – hAPP β-peptide residues 1-42 + insulin-degrading enzyme
- Amyloid precursor protein binary complex
- 1ze9 - hAPP zinc-binding domain + Zn – NMR
- 2fk1, 2fk2, 2fk3, 2fkl - hAPP residues 133-189 + Cu
- 3jti, 3gci – hAPP peptide + phospholipase A2
- 3l81 - hAPP peptide + AP-4 complex subunit μ-1
- 3ifl, 3ifn, 3ifo, 3ifp, 2r0w - hAPP peptide + antibody
- 2wk3 - hAPP residues 1-42 + insulin degrading enzyme
- 3dxc - hAPP residues 739-770 + Fe65-PTB2
- 3dxd, 3dxe - hAPP residues 739-770 (mutant) + Fe65-PTB2
- 2roz - hAPP peptide + Fe65 C terminal
- 2otk - hAPP residues 672-711 + ZAB3 affibody dimer - NMR
- 3l3t - hAPP residues 211-267 + mesotrypsin
- 3l33 - hAPP residues 290-341 + trypsin-3 (mutant)