1z3a
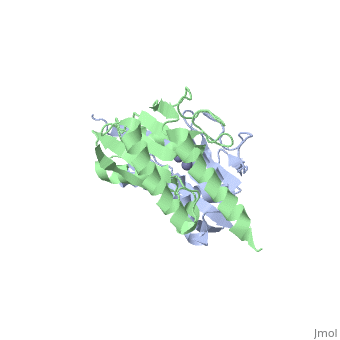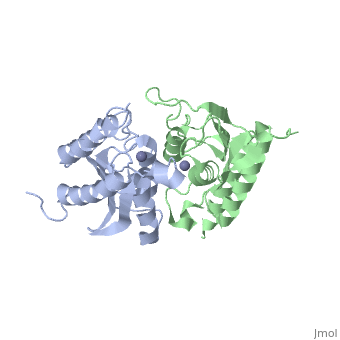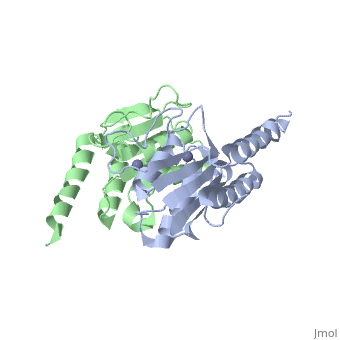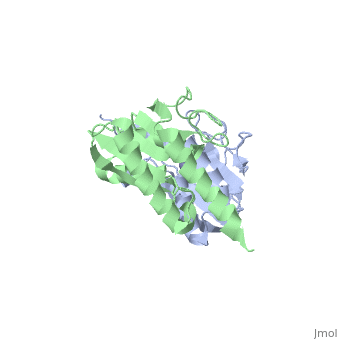
Crystal structure of tRNA adenosine deaminase TadA from Escherichia coliCrystal structure of tRNA adenosine deaminase TadA from Escherichia coli
Structural highlights
Function[TADA_ECOLI] Deaminates adenosine-34 to inosine in tRNA-Arg2. Mutation in this protein makes E.coli resistant to the toxic proteins encoded by the gef gene family. Essential for cell viability.[1] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe essential tRNA-specific adenosine deaminase catalyzes the deamination of adenosine to inosine at the wobble position of tRNAs. This modification allows for a single tRNA species to recognize multiple synonymous codons containing A, C, or U in the last (3'-most) position and ensures that all sense codons are appropriately decoded. We report the first combined structural and kinetic characterization of a wobble-specific deaminase. The structure of the Escherichia coli enzyme clearly defines the dimer interface and the coordination of the catalytically essential zinc ion. The structure also identifies the nucleophilic water and highlights residues near the catalytic zinc likely to be involved in recognition and catalysis of polymeric RNA substrates. A minimal 19 nucleotide RNA stem substrate has permitted the first steady-state kinetic characterization of this enzyme (k(cat) = 13 +/- 1 min(-)(1) and K(M) = 0.83 +/- 0.22 microM). A continuous coupled assay was developed to follow the reaction at high concentrations of polynucleotide substrates (>10 microM). This work begins to define the chemical and structural determinants responsible for catalysis and substrate recognition and lays the foundation for detailed mechanistic analysis of this essential enzyme. Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase.,Kim J, Malashkevich V, Roday S, Lisbin M, Schramm VL, Almo SC Biochemistry. 2006 May 23;45(20):6407-16. PMID:16700551[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||