Spindlin: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) New page: <StructureSection load='4mzg' size='340' side='right' caption='Caption for this structure' scene=''> == Function == '''Spindlin-1''' (SPIN1) is a Tudor-like domain-containing protein i... |
Michal Harel (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
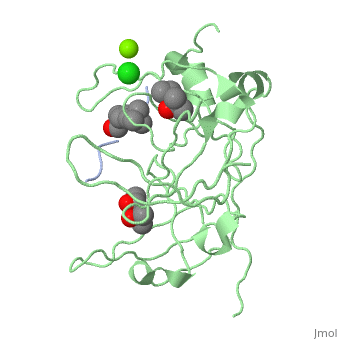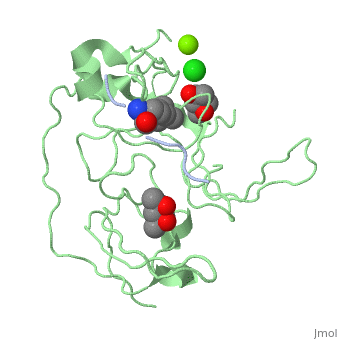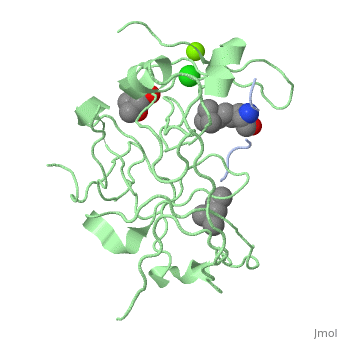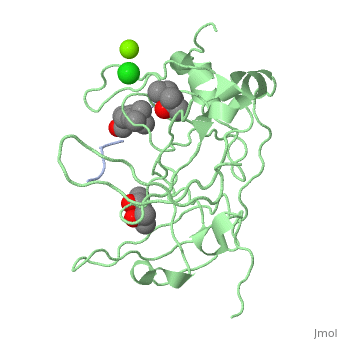
<StructureSection load='4mzg' size='340' side='right' caption='Human spindlin-1 (green) complex with histone H3 peptide (grey) containing trimethyl lysine, MPD, Mg+2 (green) and Cl- (green) ions (PDB code [[4mzg]])' scene=''> | |||
<StructureSection load='4mzg' size='340' side='right' caption=' | |||
| Line 13: | Line 12: | ||
== Structural highlights == | == Structural highlights == | ||
The crystal complex of SPIN1 and histone H3 peptide shows the encapsulation of the histone trimethyllysine and its Arg in hydrophobic pockets of SPIN1<ref>PMID:24589551</ref>. | |||
</StructureSection> | </StructureSection> | ||
== 3D Structures of spindlin == | == 3D Structures of spindlin == | ||
Revision as of 12:14, 15 January 2018
FunctionSpindlin-1 (SPIN1) is a Tudor-like domain-containing protein is a histone methylation effector protein and facilitates the expression of rRNA genes[1]. SPIN1 senses a cis-tail histone H3 methylation pattern [2]. Spindlin-2B is involved in the regulation of cell cycle progression. RelevanceSPIN1 is implicated in liposarcoma and its inhibitors may represent a novel therapeutic strategy[3]. Structural highlightsThe crystal complex of SPIN1 and histone H3 peptide shows the encapsulation of the histone trimethyllysine and its Arg in hydrophobic pockets of SPIN1[4]. |
| ||||||||||
3D Structures of spindlin3D Structures of spindlin
Updated on 15-January-2018
ReferencesReferences
- ↑ Wang W, Chen Z, Mao Z, Zhang H, Ding X, Chen S, Zhang X, Xu R, Zhu B. Nucleolar protein Spindlin1 recognizes H3K4 methylation and stimulates the expression of rRNA genes. EMBO Rep. 2011 Oct 28;12(11):1160-6. doi: 10.1038/embor.2011.184. PMID:21960006 doi:http://dx.doi.org/10.1038/embor.2011.184
- ↑ Su X, Zhu G, Ding X, Lee SY, Dou Y, Zhu B, Wu W, Li H. Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1. Genes Dev. 2014 Mar 15;28(6):622-36. doi: 10.1101/gad.233239.113. Epub 2014 Mar, 3. PMID:24589551 doi:http://dx.doi.org/10.1101/gad.233239.113
- ↑ Franz H, Greschik H, Willmann D, Ozretic L, Jilg CA, Wardelmann E, Jung M, Buettner R, Schule R. The histone code reader SPIN1 controls RET signaling in liposarcoma. Oncotarget. 2015 Mar 10;6(7):4773-89. doi: 10.18632/oncotarget.3000. PMID:25749382 doi:http://dx.doi.org/10.18632/oncotarget.3000
- ↑ Su X, Zhu G, Ding X, Lee SY, Dou Y, Zhu B, Wu W, Li H. Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1. Genes Dev. 2014 Mar 15;28(6):622-36. doi: 10.1101/gad.233239.113. Epub 2014 Mar, 3. PMID:24589551 doi:http://dx.doi.org/10.1101/gad.233239.113