4mzg
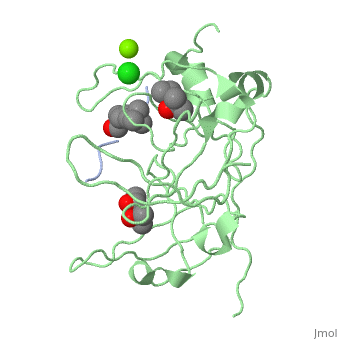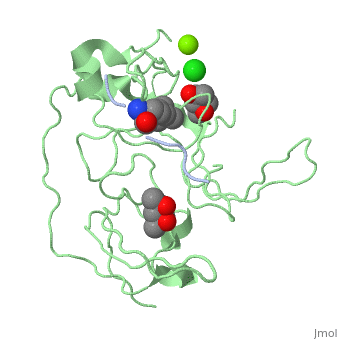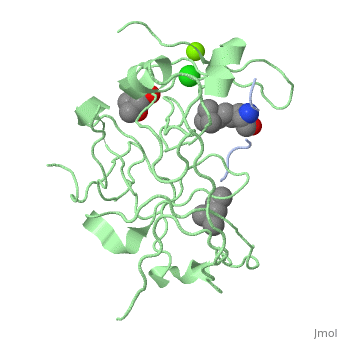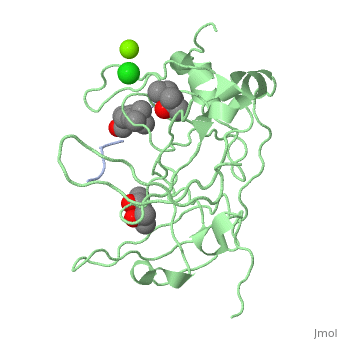
Crystal structure of human Spindlin1 bound to histone H3K4me3 peptideCrystal structure of human Spindlin1 bound to histone H3K4me3 peptide
Structural highlights
FunctionPublication Abstract from PubMedHistone modification patterns and their combinatorial readout have emerged as a fundamental mechanism for epigenetic regulation. Here we characterized Spindlin1 as a histone effector that senses a cis-tail histone H3 methylation pattern involving trimethyllysine 4 (H3K4me3) and asymmetric dimethylarginine 8 (H3R8me2a) marks. Spindlin1 consists of triple tudor-like Spin/Ssty repeats. Cocrystal structure determination established concurrent recognition of H3K4me3 and H3R8me2a by Spin/Ssty repeats 2 and 1, respectively. Both H3K4me3 and H3R8me2a are recognized using an "insertion cavity" recognition mode, contributing to a methylation state-specific layer of regulation. In vivo functional studies suggest that Spindlin1 activates Wnt/beta-catenin signaling downstream from protein arginine methyltransferase 2 (PRMT2) and the MLL complex, which together are capable of generating a specific H3 "K4me3-R8me2a" pattern. Mutagenesis of Spindlin1 reader pockets impairs activation of Wnt target genes. Taken together, our work connects a histone "lysine-arginine" methylation pattern readout by Spindlin1-to-Wnt signaling at the transcriptional level. Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1.,Su X, Zhu G, Ding X, Lee SY, Dou Y, Zhu B, Wu W, Li H Genes Dev. 2014 Mar 15;28(6):622-36. doi: 10.1101/gad.233239.113. Epub 2014 Mar, 3. PMID:24589551[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||