5tkh: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='5tkh' size='340' side='right' caption='[[5tkh]], [[Resolution|resolution]] 1.20Å' scene=''> | <StructureSection load='5tkh' size='340' side='right' caption='[[5tkh]], [[Resolution|resolution]] 1.20Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[5tkh]] is a 2 chain structure. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=5TKH OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5TKH FirstGlance]. <br> | <table><tr><td colspan='2'>[[5tkh]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Chrysonilia_crassa Chrysonilia crassa]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=5TKH OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5TKH FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CU:COPPER+(II)+ION'>CU</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=OXY:OXYGEN+MOLECULE'>OXY</scene>, <scene name='pdbligand=PER:PEROXIDE+ION'>PER</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CU:COPPER+(II)+ION'>CU</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=OXY:OXYGEN+MOLECULE'>OXY</scene>, <scene name='pdbligand=PER:PEROXIDE+ION'>PER</scene></td></tr> | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[5tki|5tki]], [[5tkf|5tkf]], [[5tkg|5tkg]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[5tki|5tki]], [[5tkf|5tkf]], [[5tkg|5tkg]]</td></tr> | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">G15G9.090, GE21DRAFT_7469 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=5141 Chrysonilia crassa])</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5tkh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=5tkh OCA], [http://pdbe.org/5tkh PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=5tkh RCSB], [http://www.ebi.ac.uk/pdbsum/5tkh PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=5tkh ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5tkh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=5tkh OCA], [http://pdbe.org/5tkh PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=5tkh RCSB], [http://www.ebi.ac.uk/pdbsum/5tkh PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=5tkh ProSAT]</span></td></tr> | ||
</table> | </table> | ||
| Line 21: | Line 22: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: Chrysonilia crassa]] | |||
[[Category: Dell, W B.O]] | [[Category: Dell, W B.O]] | ||
[[Category: Meilleur, F]] | [[Category: Meilleur, F]] | ||
[[Category: Oxidoreductase]] | [[Category: Oxidoreductase]] | ||
[[Category: Polysaccharide monooxygenase]] | [[Category: Polysaccharide monooxygenase]] | ||
Revision as of 22:13, 15 November 2017
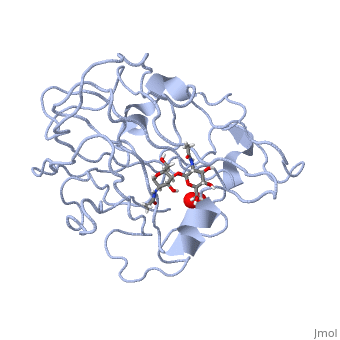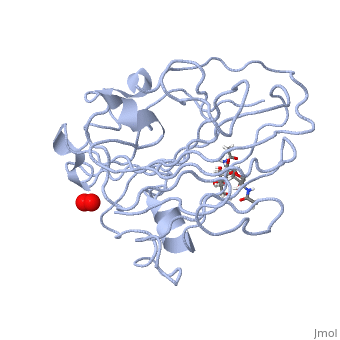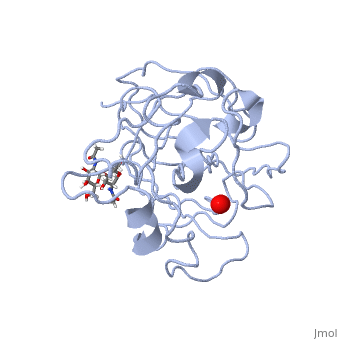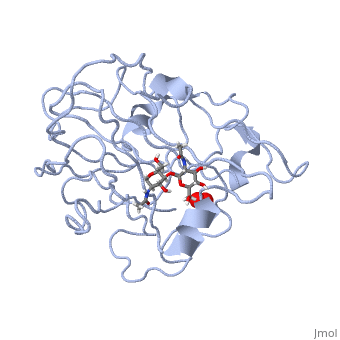
Neurospora crassa polysaccharide monooxygenase 2 ascorbate treatedNeurospora crassa polysaccharide monooxygenase 2 ascorbate treated
Structural highlights
Publication Abstract from PubMedLytic polysaccharide monooxygenases have attracted vast attention owing to their abilities to disrupt glycosidic bonds via oxidation instead of hydrolysis and to enhance enzymatic digestion of recalcitrant substrates including chitin and cellulose. We have determined high-resolution X-ray crystal structures of an enzyme from Neurospora crassa in the resting state and of a copper(II) dioxo intermediate complex formed in the absence of substrate. X-ray crystal structures also revealed "pre-bound" molecular oxygen adjacent to the active site. An examination of protonation states enabled by neutron crystallography and density functional theory calculations identified a role for a conserved histidine in promoting oxygen activation. These results provide a new structural description of oxygen activation by substrate free lytic polysaccharide monooxygenases and provide insights that can be extended to reactivity in the enzyme-substrate complex. Oxygen Activation at the Active Site of a Fungal Lytic Polysaccharide Monooxygenase.,O'Dell WB, Agarwal PK, Meilleur F Angew Chem Int Ed Engl. 2017 Jan 16;56(3):767-770. doi: 10.1002/anie.201610502., Epub 2016 Dec 22. PMID:28004877[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. References
|
| ||||||||||||||||||||