Vinculin: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| Line 17: | Line 17: | ||
**[[1tr2]] – hVCL - human<br /> | **[[1tr2]] – hVCL - human<br /> | ||
**[[1qkr]] – hVCL | **[[1qkr]] – hVCL tail domain<br /> | ||
**[[3h2v]] - hVCL | **[[3h2v]] - hVCL tail domain + raver1 RRM<br /> | ||
**[[4pr9]] - hVCL | **[[4pr9]] - hVCL tail domain + lipid<br /> | ||
**[[3jbi]] - hVCL tail domain + actin – Cryo-EM<br /> | |||
**[[3h2u]] – hVCL Vd1 + raver1 RRM <br /> | **[[3h2u]] – hVCL Vd1 + raver1 RRM <br /> | ||
**[[2ibf]], [[2hsq]], [[2gww]] - hVCL Vd1 + SfVCL binding sites from ''Shigella flexneri''<br /> | **[[2ibf]], [[2hsq]], [[2gww]] - hVCL Vd1 + SfVCL binding sites from ''Shigella flexneri''<br /> | ||
| Line 30: | Line 31: | ||
**[[3myi]] – m-VCL tail domain <br /> | **[[3myi]] – m-VCL tail domain <br /> | ||
**[[5l0f]], [[5l0i]], [[5l0 - hm-VCL tail domain (mutant)<br /> | |||
**[[5l0c]], [[5l0d]] - hm-VCL tail domain + lipid<br /> | |||
**[[5l0g]], [[5l0h]] - hm-VCL tail domain (mutant) + lipid<br /> | |||
**[[3jbk]] – hm-VCL tail domain + actin – Cryo-EM<br /> | |||
**[[3rf3]] - hm-VCL + invasin IPAA<br /> | **[[3rf3]] - hm-VCL + invasin IPAA<br /> | ||
**[[3s90]] - hm-VCL head domain + mTalin-1 peptide<br /> | **[[3s90]] - hm-VCL head domain + mTalin-1 peptide<br /> | ||
Revision as of 12:48, 13 December 2016
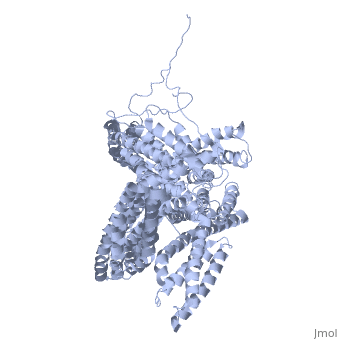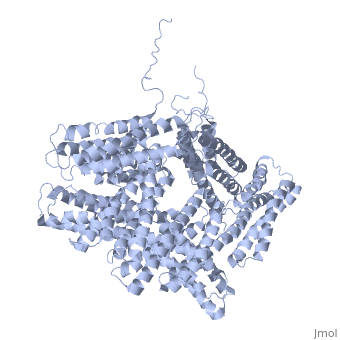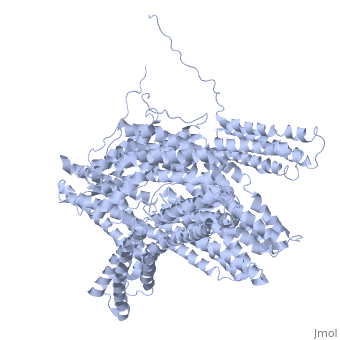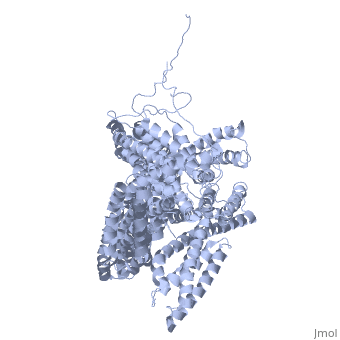
FunctionVinculins (VCLs) are involved in adhesion by linking integrin molecules to the actin cytoskeleton. Its head domain (Vd1) can bind to talin or to alpha-actinin at their respective VCL Binding Sites (VBS)[1]. The protein raver1 RNA Recognition Motif (RRM) forms a complex with VCL or m-VCL. Metavinculin (m-VCL) is a splice version of VCL containing an extra ca. 70 amino acids in the C-terminal domain. RelevanceLoss of VCL could be used as a prognostic factor for colorectal cancer se it promotes metastasis[2]. Structural highlightsis achieved through a high affinity intramolecular interaction between tail (orange) and head (aqua) domains (1st6). Energetically, I997 is key to maintaining this autoinhibition. |
| ||||||||||
3D Structures of Vinculin3D Structures of Vinculin
Updated on 13-December-2016 {{#tree:id=OrganizedByTopic|openlevels=0|
- Vinculin
- 1tr2 – hVCL - human
- 1qkr – hVCL tail domain
- 3h2v - hVCL tail domain + raver1 RRM
- 4pr9 - hVCL tail domain + lipid
- 3jbi - hVCL tail domain + actin – Cryo-EM
- 3h2u – hVCL Vd1 + raver1 RRM
- 2ibf, 2hsq, 2gww - hVCL Vd1 + SfVCL binding sites from Shigella flexneri
- 1ydi - hVCL Vd1+hActinin VBS
- 1t01 - cVCL Vd1+mTalin VBS – chicken
- 1syq, 1rkc, 1rke – hVCL Vd1+hTalin VBS
- 3zdl – cVCL Vd1 + amyloid β precursor protein N terminal
- 1tr2 – hVCL - human
- Metavinculin
- 3myi – m-VCL tail domain
- 5l0f, 5l0i, [[5l0 - hm-VCL tail domain (mutant)
- 5l0c, 5l0d - hm-VCL tail domain + lipid
- 5l0g, 5l0h - hm-VCL tail domain (mutant) + lipid
- 3jbk – hm-VCL tail domain + actin – Cryo-EM
- 3rf3 - hm-VCL + invasin IPAA
- 3s90 - hm-VCL head domain + mTalin-1 peptide
- 4dj9 - hm-VCL head domain + hTalin-1 peptide
- 3tj5 - hm-VCL head domain + Sca-family protein peptide
- 3tj6 - hm-VCL head domain + protein Ps 120 peptide
- 4ehp - hm-VCL head domain + catenin α-1 residues 277-382
- 3vf0 – hm-VCL residues 856-1134 + ribonucleoprotein PTB-binding
- 2gdc – cm-VCL Vd1+SfInvasin C-terminal
- 1xwj - cm-VCL Vd1+cTalin VBS3
- 1zvz, 1zw2, 1zw3, 1u6h - cm-VCL Vd1+cTalin rod
- 1st6 – cm-VCL
- 4e17, 4e18 - cm-VCL Vd1 + catenin α-1 VCL-binding domain
- 3myi – m-VCL tail domain
}}
ReferencesReferences
- ↑ Palovuori R, Eskelinen S. Role of vinculin in the maintenance of cell-cell contacts in kidney epithelial MDBK cells. Eur J Cell Biol. 2000 Dec;79(12):961-74. PMID:11152287 doi:http://dx.doi.org/10.1078/0171-9335-00120
- ↑ Li T, Guo H, Song Y, Zhao X, Shi Y, Lu Y, Hu S, Nie Y, Fan D, Wu K. Loss of vinculin and membrane-bound beta-catenin promotes metastasis and predicts poor prognosis in colorectal cancer. Mol Cancer. 2014 Dec 11;13:263. doi: 10.1186/1476-4598-13-263. PMID:25496021 doi:http://dx.doi.org/10.1186/1476-4598-13-263
- Created with the participation of Susan Craig.