Sandbox Reserved 478: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
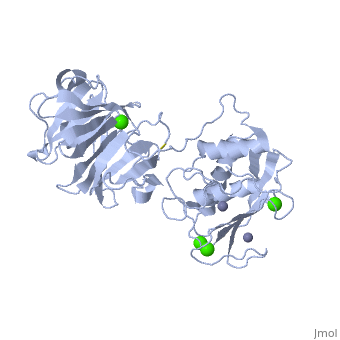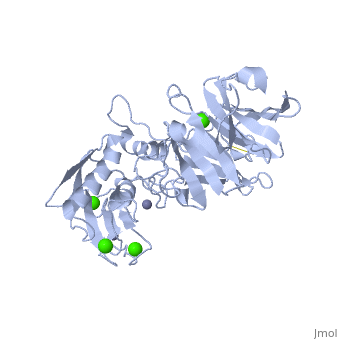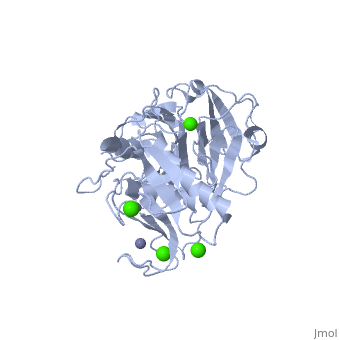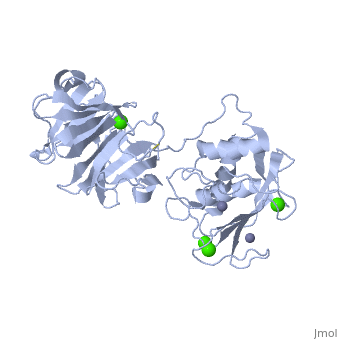
'''Matrix Metalloproteinase 1'''(MMP1) also known as Interstitial collagenase is a zinc-dependent protease that degrades extracellular matrix proteins.<ref>Nagase, H., Barrett, A. J., Woessner, J. F., Jr. Nomenclature and glossary of the matrix metalloproteinases. Matrix Suppl. 1: 421-424, 1992.</ref> [http://en.wikipedia.org/wiki/Collagenase Collagenases] are enzymes, which cleave bonds in collagen. MMP1 belongs to the Matrix Metalloproteinase (MMP) family, which are involved in the regulation of cell-matrix composition by the breakdown of the extracellular matrix in normal physiological processes. These physiological processes many include disease activities such as arthritis and metastasis as well as normal human reproduction and embryonic development.<ref>Visse, Robert, and Hideaki Nagase. "Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases." Circulation Research 92 (2003): 827-39.</ref> | '''Matrix Metalloproteinase 1'''(MMP1) also known as Interstitial collagenase is a zinc-dependent protease that degrades extracellular matrix proteins.<ref name="Nagase">Nagase, H., Barrett, A. J., Woessner, J. F., Jr. Nomenclature and glossary of the matrix metalloproteinases. Matrix Suppl. 1: 421-424, 1992.</ref> [http://en.wikipedia.org/wiki/Collagenase Collagenases] are enzymes, which cleave bonds in collagen. MMP1 belongs to the Matrix Metalloproteinase (MMP) family, which are involved in the regulation of cell-matrix composition by the breakdown of the extracellular matrix in normal physiological processes. These physiological processes many include disease activities such as arthritis and metastasis as well as normal human reproduction and embryonic development.<ref name="Visse">Visse, Robert, and Hideaki Nagase. "Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases." Circulation Research 92 (2003): 827-39.</ref> | ||
{{STRUCTURE_2clt| PDB=2clt | SCENE= }} | {{STRUCTURE_2clt| PDB=2clt | SCENE= }} | ||
==Structure== | ==Structure== | ||
| Line 20: | Line 20: | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
Several Studies have been conducted on the specific mechanism of MMPs, and many different perspectives of the mechanism have been published. The difference in the proposed mechanisms shows that the research within this area is still undergoing and there is no final verdict as to the mechanism of action. Robert Visse et al, states in a review of Matrix Metalloproteinases, that MMPs can be activated by proteinases or in vitro by chemical agents, such as thiol-modifying agents (4-aminophenylmercuric acetate, HgCl2, and N-ethylmaleimide), oxidized glutathione, SDS, chaotropic agents, and reactive oxygens. These agents mostly work to disturb the interaction between the cysteine-zinc of the cysteine switch. Proteolytic activation of MMPs is stepwise with the initial proteolytic attack occuring at an exposed loop region between the first and the second helices of the propeptide, once a portion of the propetide is removed, then the rest of the propeptide is destabilized which allows for the intermolecular processing by partially activated MMP intermediates or other MMPs. The final step in the activation process is therefore conducted by an MMP | Several Studies have been conducted on the specific mechanism of MMPs, and many different perspectives of the mechanism have been published. The difference in the proposed mechanisms shows that the research within this area is still undergoing and there is no final verdict as to the mechanism of action. Robert Visse et al, states in a review of Matrix Metalloproteinases, that MMPs can be activated by proteinases or in vitro by chemical agents, such as thiol-modifying agents (4-aminophenylmercuric acetate, HgCl2, and N-ethylmaleimide), oxidized glutathione, SDS, chaotropic agents, and reactive oxygens. <ref name="Visse" /> These agents mostly work to disturb the interaction between the cysteine-zinc of the cysteine switch. Proteolytic activation of MMPs is stepwise with the initial proteolytic attack occuring at an exposed loop region between the first and the second helices of the propeptide, once a portion of the propetide is removed, then the rest of the propeptide is destabilized which allows for the intermolecular processing by partially activated MMP intermediates or other MMPs. The final step in the activation process is therefore conducted by an MMP | ||
This image is a representation of the mode of activation that is utilized in MMP-1. the image is used to show activation in human colleganase (proMMP-1)[[Image:F3large.jpg]] | This image is a representation of the mode of activation that is utilized in MMP-1. the image is used to show activation in human colleganase (proMMP-1)[[Image:F3large.jpg]] | ||
Revision as of 06:26, 3 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. |
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Structure
|