1cfc: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
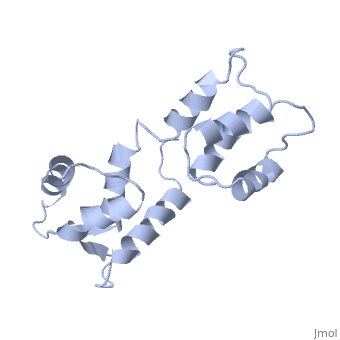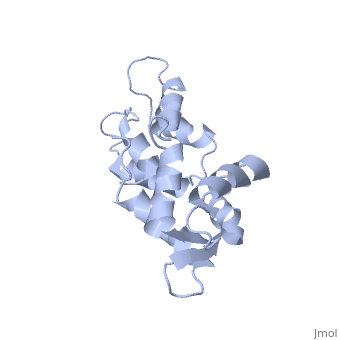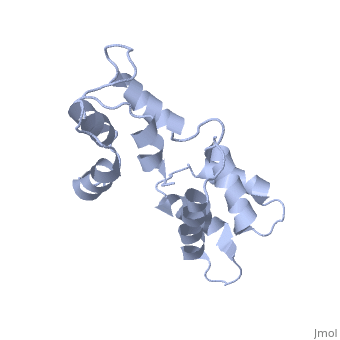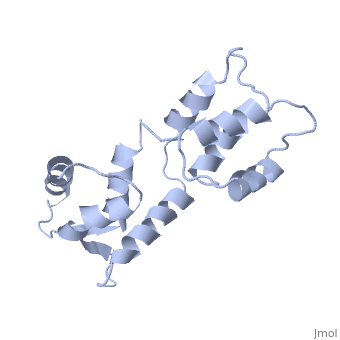
<StructureSection load='1cfc' size='340' side='right'caption='[[1cfc]], [[NMR_Ensembles_of_Models | 25 NMR models]]' scene=''> | <StructureSection load='1cfc' size='340' side='right'caption='[[1cfc]], [[NMR_Ensembles_of_Models | 25 NMR models]]' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1cfc]] is a 1 chain structure with sequence from [ | <table><tr><td colspan='2'>[[1cfc]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Xenopus_laevis Xenopus laevis]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CFC OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1CFC FirstGlance]. <br> | ||
</td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1cfd|1cfd]]</td></tr> | </td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat"><div style='overflow: auto; max-height: 3em;'>[[1cfd|1cfd]]</div></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1cfc FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1cfc OCA], [https://pdbe.org/1cfc PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1cfc RCSB], [https://www.ebi.ac.uk/pdbsum/1cfc PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1cfc ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
[[ | [[https://www.uniprot.org/uniprot/CALM_XENLA CALM_XENLA]] Calmodulin mediates the control of a large number of enzymes, ion channels and other proteins by Ca(2+). Among the enzymes to be stimulated by the calmodulin-Ca(2+) complex are a number of protein kinases and phosphatases. | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 33: | Line 33: | ||
*[[Calmodulin 3D structures|Calmodulin 3D structures]] | *[[Calmodulin 3D structures|Calmodulin 3D structures]] | ||
*[[Hydrogen in macromolecular models|Hydrogen in macromolecular models]] | *[[Hydrogen in macromolecular models|Hydrogen in macromolecular models]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 13:43, 19 May 2021
CALCIUM-FREE CALMODULINCALCIUM-FREE CALMODULIN
Structural highlights
Function[CALM_XENLA] Calmodulin mediates the control of a large number of enzymes, ion channels and other proteins by Ca(2+). Among the enzymes to be stimulated by the calmodulin-Ca(2+) complex are a number of protein kinases and phosphatases. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe three-dimensional structure of calmodulin in the absence of Ca2+ has been determined by three- and four-dimensional heteronuclear NMR experiments, including ROE, isotope-filtering combined with reverse labelling, and measurement of more than 700 three-bond J-couplings. In analogy with the Ca(2+)-ligated state of this protein, it consists of two small globular domains separated by a flexible linker, with no stable, direct contacts between the two domains. In the absence of Ca2+, the four helices in each of the two globular domains form a highly twisted bundle, capped by a short anti-parallel beta-sheet. This arrangement is qualitatively similar to that observed in the crystal structure of the Ca(2+)-free N-terminal domain of troponin C. Solution structure of calcium-free calmodulin.,Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A Nat Struct Biol. 1995 Sep;2(9):768-76. PMID:7552748[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||