1zro: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
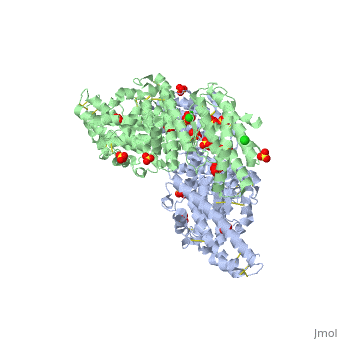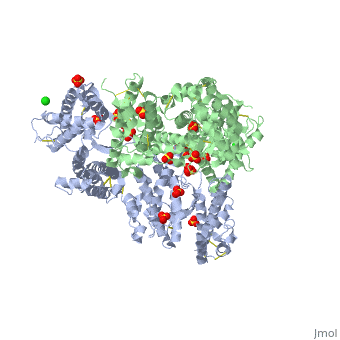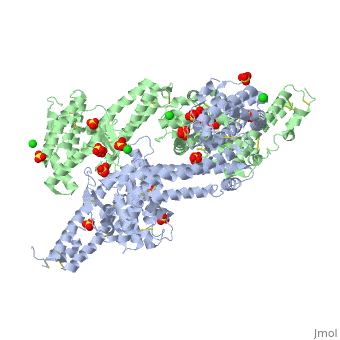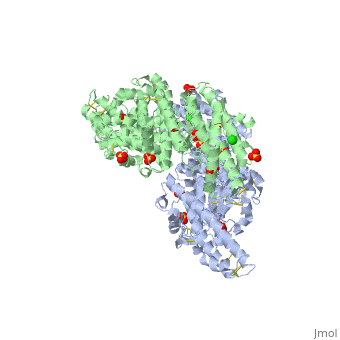
<StructureSection load='1zro' size='340' side='right' caption='[[1zro]], [[Resolution|resolution]] 2.25Å' scene=''> | <StructureSection load='1zro' size='340' side='right' caption='[[1zro]], [[Resolution|resolution]] 2.25Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1zro]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[1zro]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Plafa Plafa]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1ZRO OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1ZRO FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1zrl|1zrl]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1zrl|1zrl]]</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1zro FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1zro OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1zro RCSB], [http://www.ebi.ac.uk/pdbsum/1zro PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1zro FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1zro OCA], [http://pdbe.org/1zro PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1zro RCSB], [http://www.ebi.ac.uk/pdbsum/1zro PDBsum]</span></td></tr> | ||
</table> | </table> | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
| Line 25: | Line 25: | ||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
</div> | </div> | ||
<div class="pdbe-citations 1zro" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
| Line 32: | Line 33: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Plafa]] | ||
[[Category: Enemark, E J]] | [[Category: Enemark, E J]] | ||
[[Category: Joshua-Tor, L]] | [[Category: Joshua-Tor, L]] | ||
Revision as of 08:27, 11 September 2015
Crystal structure of EBA-175 Region II (RII) crystallized in the presence of (alpha)2,3-sialyllactoseCrystal structure of EBA-175 Region II (RII) crystallized in the presence of (alpha)2,3-sialyllactose
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedErythrocyte binding antigen 175 (EBA-175) is a P. falciparum protein that binds the major glycoprotein found on human erythrocytes, glycophorin A, during invasion. Here we present the crystal structure of the erythrocyte binding domain of EBA-175, RII, which has been established as a vaccine candidate. Binding sites for the heavily sialylated receptor glycophorin A are proposed based on a complex of RII with a glycan that contains the essential components required for binding. The dimeric organization of RII displays two prominent channels that contain four of the six observed glycan binding sites. Each monomer consists of two Duffy binding-like (DBL) domains (F1 and F2). F2 more prominently lines the channels and makes the majority of the glycan contacts, underscoring its role in cytoadherence and in antigenic variation in malaria. Our studies provide insight into the mechanism of erythrocyte invasion by the malaria parasite and aid in rational drug design and vaccines. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum.,Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L Cell. 2005 Jul 29;122(2):183-93. PMID:16051144[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||