1l6j: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
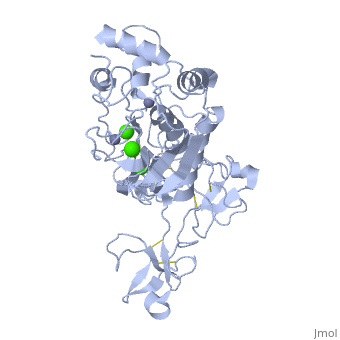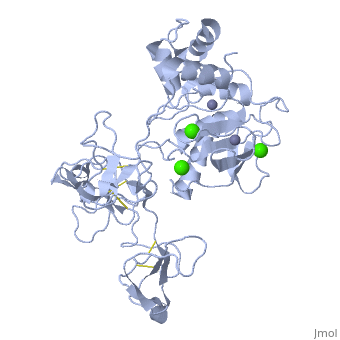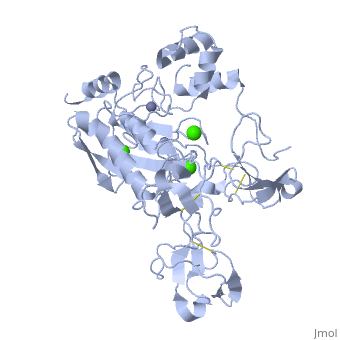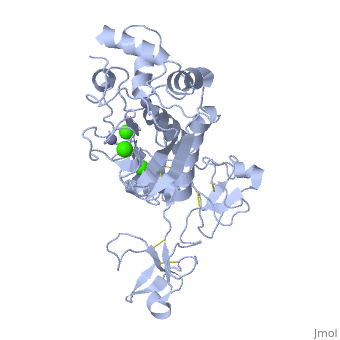
The X-ray crystal structure of the proform of human matrix | The X-ray crystal structure of the proform of human matrix metalloproteinase MMP9 has been solved to 2.5 A resolution. The construct includes the prodomain, the catalytic domain and three FnII (fibronectin type II) domains. The prodomain is inserted into the active-site cleft, blocking access to the catalytic zinc. Comparison with the crystal structure of the most closely related MMP, MMP2, indicates that the conformations of residues in the active-site cleft and in the cysteine-switch peptide of the prodomain are highly conserved and that design of MMP9-specific inhibitors will be challenging. In common with MMP2, the MMP9 S1' inhibitor-binding pocket is large compared with that of other MMPs. One small point of difference in the S1' binding pockets of MMP9 and MMP2 may provide an opportunity to explore the design of specific inhibitors. The side chain of Arg424 in MMP9 is angled slightly away from the S1' pocket when compared with the corresponding residue in MMP2, Thr424. The secondary structure of the FnII domains is conserved between the two closely related MMPs, although the second FnII domain makes no contact with the catalytic domain in MMP9, while the same domain in MMP2 has a substantial area of interaction with the catalytic domain. | ||
==About this Structure== | ==About this Structure== | ||
| Line 14: | Line 14: | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Alessio, K | [[Category: Alessio, K J.D.]] | ||
[[Category: Cummings, M | [[Category: Cummings, M D.]] | ||
[[Category: Elkins, P | [[Category: Elkins, P A.]] | ||
[[Category: Ho, Y | [[Category: Ho, Y S.]] | ||
[[Category: Janson, C | [[Category: Janson, C A.]] | ||
[[Category: McQueney, M | [[Category: McQueney, M S.]] | ||
[[Category: Romanic, A | [[Category: Romanic, A M.]] | ||
[[Category: Smith, W | [[Category: Smith, W W.]] | ||
[[Category: CA]] | [[Category: CA]] | ||
[[Category: ZN]] | [[Category: ZN]] | ||
[[Category: twisted beta sheet flanked by helices]] | [[Category: twisted beta sheet flanked by helices]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 13:41:53 2008'' | ||
Revision as of 14:41, 21 February 2008
|
Crystal structure of human matrix metalloproteinase MMP9 (gelatinase B).
OverviewOverview
The X-ray crystal structure of the proform of human matrix metalloproteinase MMP9 has been solved to 2.5 A resolution. The construct includes the prodomain, the catalytic domain and three FnII (fibronectin type II) domains. The prodomain is inserted into the active-site cleft, blocking access to the catalytic zinc. Comparison with the crystal structure of the most closely related MMP, MMP2, indicates that the conformations of residues in the active-site cleft and in the cysteine-switch peptide of the prodomain are highly conserved and that design of MMP9-specific inhibitors will be challenging. In common with MMP2, the MMP9 S1' inhibitor-binding pocket is large compared with that of other MMPs. One small point of difference in the S1' binding pockets of MMP9 and MMP2 may provide an opportunity to explore the design of specific inhibitors. The side chain of Arg424 in MMP9 is angled slightly away from the S1' pocket when compared with the corresponding residue in MMP2, Thr424. The secondary structure of the FnII domains is conserved between the two closely related MMPs, although the second FnII domain makes no contact with the catalytic domain in MMP9, while the same domain in MMP2 has a substantial area of interaction with the catalytic domain.
About this StructureAbout this Structure
1L6J is a Single protein structure of sequence from Homo sapiens with and as ligands. Active as Gelatinase B, with EC number 3.4.24.35 Full crystallographic information is available from OCA.
ReferenceReference
Structure of the C-terminally truncated human ProMMP9, a gelatin-binding matrix metalloproteinase., Elkins PA, Ho YS, Smith WW, Janson CA, D'Alessio KJ, McQueney MS, Cummings MD, Romanic AM, Acta Crystallogr D Biol Crystallogr. 2002 Jul;58(Pt 7):1182-92. Epub 2002, Jun 20. PMID:12077439
Page seeded by OCA on Thu Feb 21 13:41:53 2008