Spectrin: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
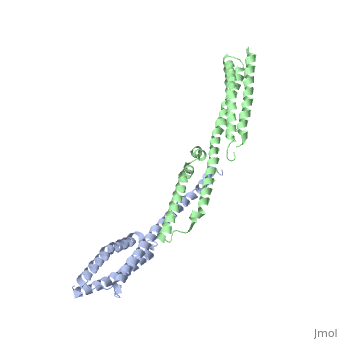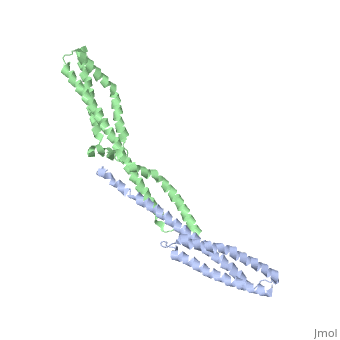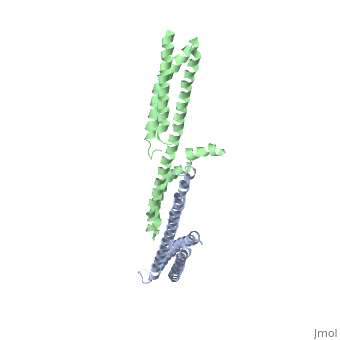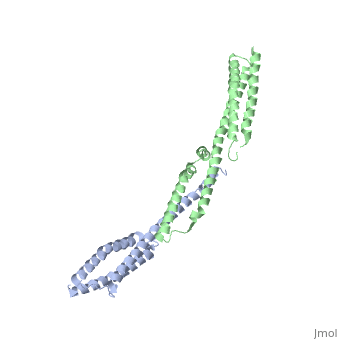
<StructureSection load='3lbx' size='340' side='right' caption='Human spectrin α (grey) and β1 chain (green) [[3lbx]]' scene=''> | |||
== Function == | |||
[[Spectrin]] forms scaffolding in plasma membranes and cytoskeletal structure. It interacts with actin at either end of its tetramer<ref>PMID:17060500</ref>. The SPT dimer is formed by association of α1 and β1 monomers. In invertebrates there are SPT α, β and βH. In vertebrates there are SPT α1 (SPTA1), α2 (SPTA2) and β1 (SPTB1) to β5. SPT contains an SRC Homology 3 domain (SH3), a Pleckstrin Homology (PH) domain and a Calponin Homology (CH) domain. | |||
*'''Spectrin α2''' is expressed highly in heart muscle cells<ref>PMID:15360127</ref>. | |||
*'''Spectrin β2''' is associated with GABA receptor at dendritic synapses<ref>PMID:36604600</ref>. | |||
*'''Spectrin β4''' is associated with GABA receptor at axon initial segment synapses. | |||
*'''Spectrin R16''' is spectrin α first repeat domain<ref>PMID:10481916</ref>. | |||
== Disease == | |||
Mutations in SPT α are found in patients with hereditary elliptocytosis<ref>PMID:2346784</ref>. SPT β deficiency is found in hereditary spherocytosis<ref>PMID:9714702</ref>. | |||
== 3D Structures of Spectrin == | == 3D Structures of Spectrin == | ||
[[Spectrin 3D structures]] | |||
</StructureSection> | |||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 09:55, 14 August 2024
FunctionSpectrin forms scaffolding in plasma membranes and cytoskeletal structure. It interacts with actin at either end of its tetramer[1]. The SPT dimer is formed by association of α1 and β1 monomers. In invertebrates there are SPT α, β and βH. In vertebrates there are SPT α1 (SPTA1), α2 (SPTA2) and β1 (SPTB1) to β5. SPT contains an SRC Homology 3 domain (SH3), a Pleckstrin Homology (PH) domain and a Calponin Homology (CH) domain.
DiseaseMutations in SPT α are found in patients with hereditary elliptocytosis[5]. SPT β deficiency is found in hereditary spherocytosis[6]. 3D Structures of Spectrin |
| ||||||||||
ReferencesReferences
- ↑ Das A, Base C, Dhulipala S, Dubreuil RR. Spectrin functions upstream of ankyrin in a spectrin cytoskeleton assembly pathway. J Cell Biol. 2006 Oct 23;175(2):325-35. PMID:17060500 doi:http://dx.doi.org/10.1083/jcb.200602095
- ↑ Bennett PM, Baines AJ, Lecomte MC, Maggs AM, Pinder JC. Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils. J Muscle Res Cell Motil. 2004;25(2):119-26. PMID:15360127 doi:10.1023/b:jure.0000035892.77399.51
- ↑ Smalley JL, Cho N, Ng SFJ, Choi C, Lemons AHS, Chaudry S, Bope CE, Dengler JS, Zhang C, Rasband MN, Davies PA, Moss SJ. Spectrin-beta 2 facilitates the selective accumulation of GABA(A) receptors at somatodendritic synapses. Commun Biol. 2023 Jan 5;6(1):11. PMID:36604600 doi:10.1038/s42003-022-04381-x
- ↑ Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999 Aug 20;98(4):523-35. PMID:10481916
- ↑ Coetzer T, Palek J, Lawler J, Liu SC, Jarolim P, Lahav M, Prchal JT, Wang W, Alter BP, Schewitz G, et al.. Structural and functional heterogeneity of alpha spectrin mutations involving the spectrin heterodimer self-association site: relationships to hematologic expression of homozygous hereditary elliptocytosis and hereditary pyropoikilocytosis. Blood. 1990 Jun 1;75(11):2235-44. PMID:2346784
- ↑ Dhermy D, Galand C, Bournier O, Cynober T, Mechinaud F, Tchemia G, Garbarz M. Hereditary spherocytosis with spectrin deficiency related to null mutations of the beta-spectrin gene. Blood Cells Mol Dis. 1998 Jun;24(2):251-61. PMID:9714702 doi:http://dx.doi.org/10.1006/bcmd.1998.0190