Stimulator of interferon genes: Difference between revisions
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (24 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
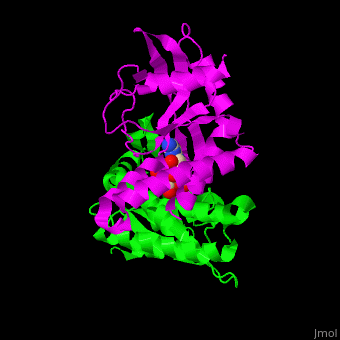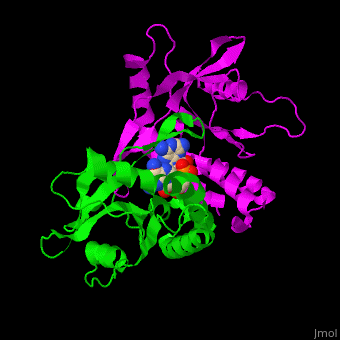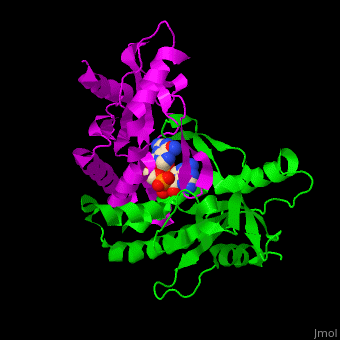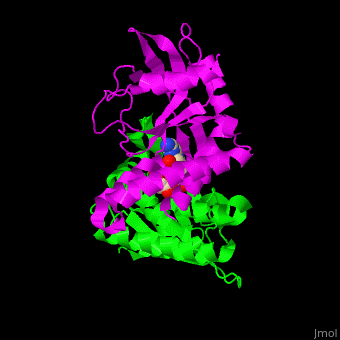
<StructureSection load=' | <StructureSection load='' size='350' side='right' caption='Structure of human STING CTD complex with c-GMP-AMP (PDB entry [[4loh]])' scene='57/573101/Cv/1'> | ||
'''Stimulator of interferon genes''' (STING) | == Function == | ||
'''Stimulator of interferon genes protein''' or '''transmembrane protein 173''' (STING) is an ER-associated membrane protein. STING signals immune responses in human and other animals. STING is activated by cyclic GMP-AMP produced by [[Cyclic GMP-AMP synthase]] whose activity is triggered by infections of DNA-containing pathogens<ref>PMID:30842659</ref>. A PLPLRT/SD conserved motif at the STING C-terminal plays a critical role in turning on the immune system to fight against viral infections<ref>PMID:31118511</ref>. | |||
== | == Disease == | ||
STING mutations are associated with autoimmune diseases<ref>PMID:26235147</ref>. | |||
== Structural highlights == | |||
The 3D structure of the complex between human STING and cyclic GMP-AMP shows the dinucleotide bound by the symmetric dimer of STING. The <scene name='57/573101/Cv/5'>U-shaped cleft</scene> between the 2 subunits makes numerous interactions with the U-shaped ligand. <scene name='57/573101/Cv/4'>GMP-AMP binding site</scene> (water molecules are shown as red spheres). A <scene name='57/573101/Cv/6'>Tyr residue from each monomer stacks</scene> against each of the purine moieties while an <scene name='57/573101/Cv/7'>Arg residue from each monomer forms hydrogen bonds to the dinucleotide</scene> <ref>PMID:23910378</ref>. | |||
==3D structures of stimulator of interferon genes protein== | |||
[[ | [[Stimulator of interferon genes protein 3D structures]] | ||
</StructureSection> | |||
== References == | |||
<references/> | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 10:29, 11 April 2022
FunctionStimulator of interferon genes protein or transmembrane protein 173 (STING) is an ER-associated membrane protein. STING signals immune responses in human and other animals. STING is activated by cyclic GMP-AMP produced by Cyclic GMP-AMP synthase whose activity is triggered by infections of DNA-containing pathogens[1]. A PLPLRT/SD conserved motif at the STING C-terminal plays a critical role in turning on the immune system to fight against viral infections[2]. DiseaseSTING mutations are associated with autoimmune diseases[3]. Structural highlightsThe 3D structure of the complex between human STING and cyclic GMP-AMP shows the dinucleotide bound by the symmetric dimer of STING. The between the 2 subunits makes numerous interactions with the U-shaped ligand. (water molecules are shown as red spheres). A against each of the purine moieties while an [4]. 3D structures of stimulator of interferon genes protein |
| ||||||||||
ReferencesReferences
- ↑ Shang G, Zhang C, Chen ZJ, Bai XC, Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature. 2019 Mar 6. pii: 10.1038/s41586-019-0998-5. doi:, 10.1038/s41586-019-0998-5. PMID:30842659 doi:http://dx.doi.org/10.1038/s41586-019-0998-5
- ↑ Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, Liu M, Lei Y, Gao X, Fu X, Zhu F, Liu Y, Laganowsky A, Zheng X, Ji JY, West AP, Watson RO, Li P. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature. 2019 May;569(7758):718-722. doi: 10.1038/s41586-019-1228-x. Epub 2019 May, 22. PMID:31118511 doi:http://dx.doi.org/10.1038/s41586-019-1228-x
- ↑ Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING Activation by Translocation from the ER Is Associated with Infection and Autoinflammatory Disease. Cell Host Microbe. 2015 Aug 12;18(2):157-68. doi: 10.1016/j.chom.2015.07.001., Epub 2015 Jul 30. PMID:26235147 doi:http://dx.doi.org/10.1016/j.chom.2015.07.001
- ↑ Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, Hartmann G, Barchet W, Tuschl T, Patel DJ. Structure-Function Analysis of STING Activation by c[G(2',5')pA(3',5')p] and Targeting by Antiviral DMXAA. Cell. 2013 Aug 15;154(4):748-62. doi: 10.1016/j.cell.2013.07.023. Epub 2013 Aug, 1. PMID:23910378 doi:10.1016/j.cell.2013.07.023