1vgj: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='1vgj' size='340' side='right'caption='[[1vgj]], [[Resolution|resolution]] 1.94Å' scene=''> | <StructureSection load='1vgj' size='340' side='right'caption='[[1vgj]], [[Resolution|resolution]] 1.94Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1vgj]] is a 1 chain structure with sequence from [ | <table><tr><td colspan='2'>[[1vgj]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Pyrococcus_horikoshii Pyrococcus horikoshii]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1VGJ OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1VGJ FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.94Å</td></tr> | ||
<tr id=' | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=MSE:SELENOMETHIONINE'>MSE</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1vgj FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1vgj OCA], [https://pdbe.org/1vgj PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1vgj RCSB], [https://www.ebi.ac.uk/pdbsum/1vgj PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1vgj ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | |||
[https://www.uniprot.org/uniprot/THPR_PYRHO THPR_PYRHO] Hydrolyzes RNA 2',3'-cyclic phosphodiester to an RNA 2'-phosphomonoester.[HAMAP-Rule:MF_01940] | |||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 34: | Line 36: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: Morita | [[Category: Pyrococcus horikoshii]] | ||
[[Category: Okada | [[Category: Morita H]] | ||
[[Category: Tanaka | [[Category: Okada A]] | ||
[[Category: Yao | [[Category: Tanaka I]] | ||
[[Category: Yao M]] | |||
Latest revision as of 10:55, 25 October 2023
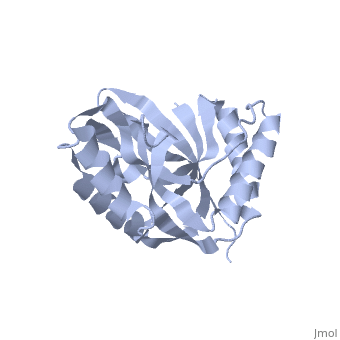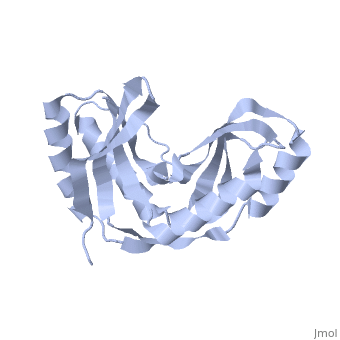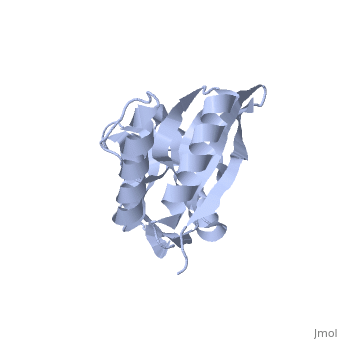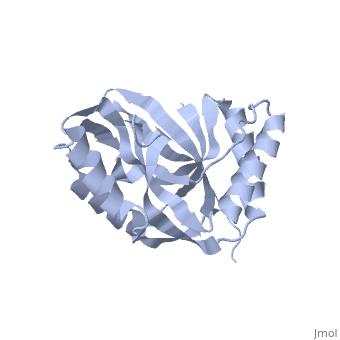
Crystal structure of 2'-5' RNA ligase from Pyrococcus horikoshiiCrystal structure of 2'-5' RNA ligase from Pyrococcus horikoshii
Structural highlights
FunctionTHPR_PYRHO Hydrolyzes RNA 2',3'-cyclic phosphodiester to an RNA 2'-phosphomonoester.[HAMAP-Rule:MF_01940] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedBacterial and archaeal 2'-5' RNA ligases, members of the 2H phosphoesterase superfamily, catalyze the linkage of the 5' and 3' exons via a 2'-5'-phosphodiester bond during tRNA-precursor splicing. The crystal structure of the 2'-5' RNA ligase PH0099 from Pyrococcus horikoshii OT3 was solved at 1.94 A resolution (PDB code 1vgj). The molecule has a bilobal alpha+beta arrangement with two antiparallel beta-sheets constituting a V-shaped active-site cleft, as found in other members of the 2H phosphoesterase superfamily. The present structure was significantly different from that determined previously at 2.4 A resolution (PDB code 1vdx) in the active-site cleft; the entrance to the cleft is wider and the active site is easily accessible to the substrate (RNA precursor) in our structure. Structural comparison with the 2'-5' RNA ligase from Thermus thermophilus HB8 also revealed differences in the RNA precursor-binding region. The structural differences in the active-site residues (tetrapeptide motifs H-X-T/S-X) between the members of the 2H phosphoesterase superfamily are discussed. The structure of Pyrococcus horikoshii 2'-5' RNA ligase at 1.94 A resolution reveals a possible open form with a wider active-site cleft.,Gao YG, Yao M, Okada A, Tanaka I Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006 Dec 1;62(Pt, 12):1196-200. Epub 2006 Nov 30. PMID:17142895[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||