Rop protein: Difference between revisions
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (31 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
<StructureSection load='1rop' size='350' side='right' scene='' caption='E. coli Rop protein [[1rop]]'> | |||
Each monomer has molecular weight of | |||
==Function== | |||
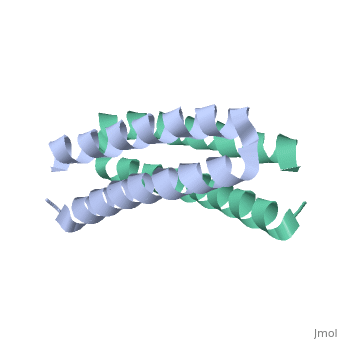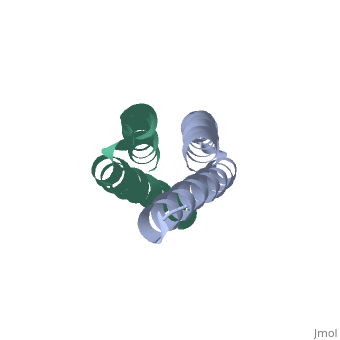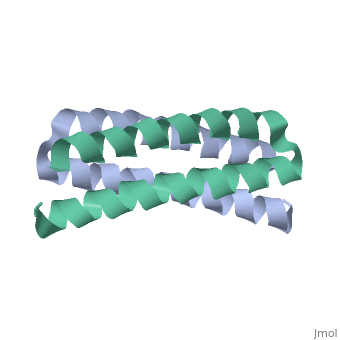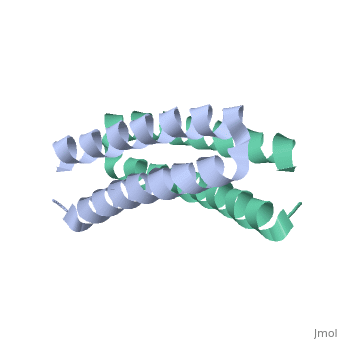
<scene name='Rop_protein/Wt_rop/1'>Rop</scene> '''(Repressor Of Primer)''' is a small homodimeric RNA-binding protein that is involved in the regulation of copy number of the ColE1 plasmids of E.coli, where it is encoded<ref>pmid 2462471</ref>. Its structure has been studied using both X-ray crystallography<ref>PMID:3681971</ref> and NMR<ref>PMID:1841691</ref>. | |||
Each monomer has molecular weight of about 7.500 Da and it consists of 63 amino acids that form two α-helices connected by <scene name='Rop_protein/Wt_rop_loop/2'> a loop </scene>of four amino acids (L29, D30, A31, D32). The two monomers are related with a 2-fold symmetry axis. | |||
==4-α-helical bundle== | ==4-α-helical bundle== | ||
Rop is the paradigm of a canonical 4-α-helical bundle and its apparent structural simplicity of its folding rendered it as model system | Rop is the paradigm of a canonical 4-α-helical bundle and its apparent structural simplicity of its folding rendered it as a model system for investigating the sequence-structure relationships in the folding and dynamics of 4-α-helix. The four α-helices are amphipathic, packed in an antiparallel fashion and display a specific pattern of hydrophobic and hydrophilic amino acids, of the type (a,b,c,d,e,f,g)n, which is repeated every seven residues (heptad pattern). Positions a and d are generally hydrophobic and the side chains of these residues are packed in the central part of the structure according to the “knobs in holes” model forming the hydrophobic core. The heptad periodicity in the sequence of Rop is disrupted only once and leads to the formation of the loop. <scene name='Rop_protein/Wt_rop_a31/2'>Ala31</scene> has a crucial role in the formation of the loop region as it is the only amino acid that simultaneously forms hydrogen bond to both helices. | ||
== Mutants == | == Mutants == | ||
Numerous mutations in the loop region of Rop have been produced | Numerous mutations in the loop region of Rop have been produced: | ||
*deletion of 5 a/a of loop ([[1qx8]]) | |||
*site-directed mutants in loop | |||
*replacement and insertion of glycine residues in loop | |||
*restored of heptad pattern in loop region) ([[1nkd]]) | |||
*a single alanine to proline substitution ([[1b6q]]) (Pro31, unlike Ala31, is more confor-mationally constrained, the dihedral angles of Ala31 in the wild type molecule are prohibited to Pro, and leads to a folding pathway for a thermodynamically less stable conformation.) | |||
*re-engineering topology of the homodimeric ROP protein into a single-chain 4-helix bundle([[1yo7]]) | |||
*ALA2ILE2-6, repacted the hydrophobic core and a new fold ([[1f4n]]) | |||
</StructureSection> | |||
==3D structures of Rop protein== | |||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | |||
[[1rpo]], [[1rop]], [[2ghy]] – EcRop – ''Escherichia coli''<br /> | |||
[[1qx8]], [[1nkd]], [[1b6q]], [[1yo7]], [[1f4n]], [[3k79]], [[2ijh]], [[2iji]], [[2ijj]], [[2ijk]], [[1gmg]], [[1f4m]], [[4do2]], [[1gto]], [[7kae]] – EcRop (mutant)<br /> | |||
[[1rpr]] – EcRop – NMR<br /> | |||
[[3q5z]] – TgRop5B pseudokinase domain – ''Toxoplasma gondii'' <br /> | |||
[[3q60]] – TgRop5B pseudokinase domain + ATP | |||
==Additional Resources== | |||
For additional information, see: [[DNA Replication, Repair, and Recombination]] | |||
<br /> | |||
==References== | |||
<references/> | |||
[[Category:Topic Page]] | |||
{{Clear}} | {{Clear}} | ||
Latest revision as of 11:14, 9 March 2022
Function(Repressor Of Primer) is a small homodimeric RNA-binding protein that is involved in the regulation of copy number of the ColE1 plasmids of E.coli, where it is encoded[1]. Its structure has been studied using both X-ray crystallography[2] and NMR[3]. Each monomer has molecular weight of about 7.500 Da and it consists of 63 amino acids that form two α-helices connected by of four amino acids (L29, D30, A31, D32). The two monomers are related with a 2-fold symmetry axis. 4-α-helical bundleRop is the paradigm of a canonical 4-α-helical bundle and its apparent structural simplicity of its folding rendered it as a model system for investigating the sequence-structure relationships in the folding and dynamics of 4-α-helix. The four α-helices are amphipathic, packed in an antiparallel fashion and display a specific pattern of hydrophobic and hydrophilic amino acids, of the type (a,b,c,d,e,f,g)n, which is repeated every seven residues (heptad pattern). Positions a and d are generally hydrophobic and the side chains of these residues are packed in the central part of the structure according to the “knobs in holes” model forming the hydrophobic core. The heptad periodicity in the sequence of Rop is disrupted only once and leads to the formation of the loop. has a crucial role in the formation of the loop region as it is the only amino acid that simultaneously forms hydrogen bond to both helices. MutantsNumerous mutations in the loop region of Rop have been produced:
|
| ||||||||||
3D structures of Rop protein3D structures of Rop protein
Updated on 09-March-2022
1rpo, 1rop, 2ghy – EcRop – Escherichia coli
1qx8, 1nkd, 1b6q, 1yo7, 1f4n, 3k79, 2ijh, 2iji, 2ijj, 2ijk, 1gmg, 1f4m, 4do2, 1gto, 7kae – EcRop (mutant)
1rpr – EcRop – NMR
3q5z – TgRop5B pseudokinase domain – Toxoplasma gondii
3q60 – TgRop5B pseudokinase domain + ATP
Additional ResourcesAdditional Resources
For additional information, see: DNA Replication, Repair, and Recombination
ReferencesReferences
- ↑ Polisky B. ColE1 replication control circuitry: sense from antisense. Cell. 1988 Dec 23;55(6):929-32. PMID:2462471
- ↑ Banner DW, Kokkinidis M, Tsernoglou D. Structure of the ColE1 rop protein at 1.7 A resolution. J Mol Biol. 1987 Aug 5;196(3):657-75. PMID:3681971
- ↑ Eberle W, Pastore A, Sander C, Rosch P. The structure of ColE1 rop in solution. J Biomol NMR. 1991 May;1(1):71-82. PMID:1841691