Sandbox Reserved 820
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Calsequestrin-2 (or CASQ2) is the soluble Ca2+ binding protein in the sarcoplasmic reticulum lumen of the cardiac muscle cells. CASQ2 could be either in a monomeric, homodimeric, or homooligomeric chain form depending on its bounds with Ca2+. Mutations of CASQ2 are involved in cardiac diseases such as Catecholaminergic Polymorphic Ventricular Tachycardia.[1] 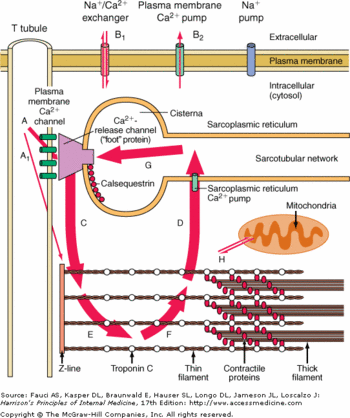 Biological roleThe contractions of cardiac myocytes are triggered by the increase of calcium concentration in the cytosol. This phenomenon is highly controlled at several levels. First the calcium is stocked in a cell compartment called the sarcoplasmic reticulum. Then the release of calcium in the cytosol is dependent of the myocytes membrane depolarization. Finally the release of calcium is extremely brief, as soon as the depolarization is over, the calcium is actively pumped in the sarcoplasmic reticulum. The calsequestrin 2 plays a major role here, because it regulates the release of the calcium in the cytosol while the membrane depolarization occurs and traps the calcium inside the lumen of the sarcoplasmic reticulum.[2] It is also good to notice that a huge release of calcium in the cytosol would be lethal to the cell, since the calcium would precipitate with the free phosphate groups.
StructureMonomere StructureEach monomer is divided in : , and the domains. Each of these has a regular structure: a surrounded by .[3] Usually these domains are involved in redox phenomena, which lead to disulfide bounds creation. Here these domains are inactive but play an important role in the polymerization of CASQ2.[4] Finally, the C-term Asp-rich end is intrisically disordered (therefore, the C-term end cannot be represented in 3D structures). [5] Polymer StructureWithin the sarcoplasmic reticulum (SR) lumen, CASQ2 polymerizes to form , homotetramers and . There are two forms of dimerization: the and the .[6] The front-to-front one is stabilized by intermolecular interactions between the of each CASQ2.[6] The intermolecular salt bridges are built between .[6] This dimerization induces the formation of an electronegative pocket which involves the following amino acids: Glu 39, Glu 54, Glu 78, Glu 92, Asp 93 and Asp 101 for the first monomere and Glu 199, Asp 245, Asp 278, Glu 348 and Glu 350 for the second one.[6] The back-to-back form is stabilized by intermolecular interactions between the , [6], and it has also been proved that the is involved[7] (). The intermolecular salt bridges are built between Glu 215 and Lys 86, Glu 216 and Lys 24, Glu 169 and Lys 85.[6] The dimerization is also favored by a hydrogen bond between Ala 82 and Asn 22. This dimerization creates a very electronegative pocket at the C-terminal region which enables the binding of Ca2+.[6]
Calcium BindingEach monomere of CASQ2 can bind between . The Ca2+ ions bind to two or more acidic amino acids like or . These amino acids are mainly oriented outside and in the C-terminal region. It had been shown that Ca2+ions mainly bind an Asp-rich region on the disordered C-terminal domain. When CASQ2 form homooligomers, Ca2+ can be bound in the electronegative pockets created by the and dimer interactions.[8] CASQ2 can also bind other ions like Mg2+ or H+. The affinity for Mg2+ is lower than the affinity for Ca2+ however the concentration of Ca2+ decreases. When the pH is low, the calcium-binding capacity of CASQ2 decreases as H+ ions occupy the acidic sites and inhibit the polymerization.[9] Interaction between CASQ2, Junctin and TriadinBinding sitesCASQ2 can be anchored into the membrane of SR thanks to two integral proteins: the triadin and the junctin.[10] Triadin and juctin can bind to CASQ2 on their KEKE motifs (amino acids 210-224 in the triadin chain).[10] Both proteins bind CASQ2 on its Asp-rich region of the C-terminal region.[10] Triadin and Junctin can also interact with Ryanodin Receptor.[10] The binding site of CASQ2 to Ryanodin Receptor (RyR) is unknown.[10] Consequences of CASQ2 binding When CASQ2 binds to triadin and junctin, it induces the inhibition of RyR and then the inhibition of calcium release in the cytoplasm. On the contrary, when CASQ2 unbinds triadin and junctin, it induces the activation of Ryr and an efflux of Ca2+ from the SR to the cytoplasm.[9] CASQ2 is free when the concentration of Ca2+ is higher than 1 mM in the SR lumen.[10]
Posttranslational modifications of CASQ2CASQ2 can be phosphorylated by three different kinases: casein kinase I (CK I), casein kinase II (CK II) and ε protein kinase C1 (εPKC1).[9] CK II is located in the SR and is able to phosphorylate Ser 378, Ser 382 and Ser 386. These residues are on the C-terminal domain.[9] The consensus sequence recognized by CK II is Ser/Thr-X-X-Asp/Glu.[9] The phosphorylation is more likely if there are acidic residues after this consensus sequence.[9] The phosphorylation and dephosphorylation of CASQ2 may provide an off/on switch for CASQ2 to regulate Ca2+ capture. But there is not any proof yet.[9] However it is known that phosphorylations on CASQ2 modify the interactions between CASQ2 and RyR but not between CASQ2 and Triadin and Junctin.[9]
|
| ||||||||||
ReferencesReferences
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ NCBI Gene Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/gene/845
- ↑ Martin JL. Thioredoxin--a fold for all reasons. Structure. 1995 Mar 15;3(3):245-50. PMID:7788290
- ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?ascbin=8&maxaln=10&seltype=2&uid=239372&querygi=429544235&aln=1,227,0,109
- ↑ Polymerization of Calsequestrin: IMPLICATIONS FOR Ca2+ and REGULATION (Park et al., 2003) http://www.jbc.org/content/278/18/16176.full.pdf+html
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html Cite error: Invalid
<ref>tag; name "Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)" defined multiple times with different content - ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi
- ↑ The Asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000) http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004 May;85(1):33-69. PMID:15050380 doi:http://dx.doi.org/10.1016/j.pbiomolbio.2003.07.001 Cite error: Invalid
<ref>tag; name "Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)" defined multiple times with different content - ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005 May;88(5):3444-54. Epub 2005 Feb 24. PMID:15731387 doi:http://dx.doi.org/10.1529/biophysj.104.051441 Cite error: Invalid
<ref>tag; name "Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)" defined multiple times with different content
Proteopedia page contributors and editorsProteopedia page contributors and editors
Marc-Antoine JACQUES and Thomas VUILLEMIN