Sandbox Reserved 492
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | ||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Cholix Toxin from Vibrio Cholerae
|
|
Essentially, the replaces what would be the target protein in the epithelial cell of a human thus prevents the Cholera Toxin from interacting with its normal ligand; this would in turn prevent the ill effects of intoxication...in theory.
Cholix also serves several valuable purposes in Biological research. Dr. Pierre-Hervé Luppi and his laboratory recently discovered a new "tracing" method using cholera-toxin instead of the previously used classical molecules such as Horse Radish Peroxidase (HRP). In contrast to HRP, which is passively taken up by neurons, cholera-toxin (as we discussed previously) binds specifically to surface receptors of neurons and is can be actively taken up and transported by the axons. [6] This phenomenon helps enhance the sensitivity of cholera-toxin as a tracer, for studies dealing with neural function and the diseases known to affect it.  Structure of PJ34
Structure of PJ34
As a result, aspirations for future research being done on Cholera Toxin today, coincide with a current "hot topic" within the science community and the entre World: Stem Cell Research. There have been some recent findings indicating that the protein may be capable of interacting - regulation on the genetic level - some key factors in Neural Stem Cell (NSC) regeneration and differentiation. Known as Tie2, a membrane receptor, and Hes3 a transcription factor, these two indicators have been shown to directly interact with the Cholix Toxin. Moreover, there are even some implications that the protein, when combined with specific medium, boosted Stem Cell culture growth.[7] Thus, we see that apart from its potential to cause human illness, CX also poses a solution to cancer and other related diseases. 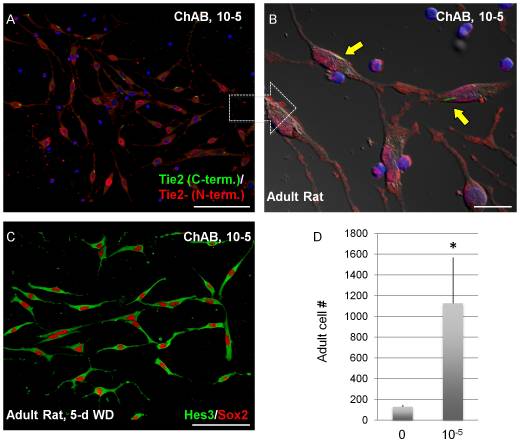
ReferencesReferences
- ↑ [1] Zhang R, Scott D, Westbrook M, Nance S, Spangler B, Shipley G, Westbrook E (1995). "The three-dimensional crystal structure of cholera toxin". J Mol Biol 251 (4): 563–73. doi:10.1006/jmbi.1995.0456.PMID 7658473.
- ↑ [2] O'Neal C, Jobling M, Holmes R, Hol W (2005). "Structural basis for the activation of cholera toxin by human ARF6-GTP". Science 309 (5737): 1093–6. doi:10.1126/science.1113398. PMID 16099990.
- ↑ [3] Joaquín Sánchez, Jan Holmgren (February 2011). [icmr.nic.in/ijmr/2011/february/0204.pdf "Cholera toxin – A foe & a friend"]. Indian Journal of Medical Research 133: p. 158.
- ↑ [4]http://www.ebi.ac.uk/interpro/potm/2005_9/Page2.htm
- ↑ [5]Jørgensen, R, A E. Purdy, R J. Fieldhouse, D H. Bartless, and A R. Merrill. “Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae..” Journal of Biological Chemistry 8, (2008)
- ↑ [6] Pierre-Hervé Luppi. "The Discovery of Cholera-Toxin as a Powerful Neuroanatomical Tool". Retrieved 2011-03-23.
- ↑ [7]Androutsellis-Theotokis, Andreas, Stuart Walbridge, Deric M. Park, Russel R. Lonser, and Ronald D. McKay. “Cholera Toxin Regulates a Signaling Pathway Critical for the Expansion of Neural Stem Cell Cultures from the Fetal and Adult Rodent Brains.” PLoS ONE 5, (2010)
