Malate synthase
Jump to navigation
Jump to search
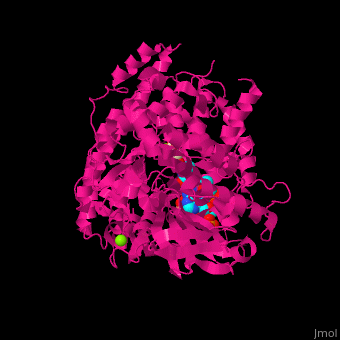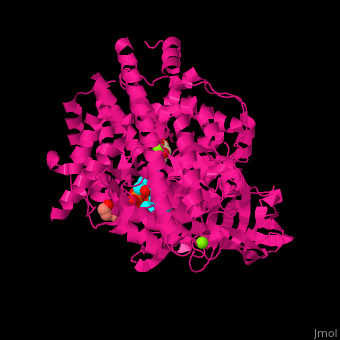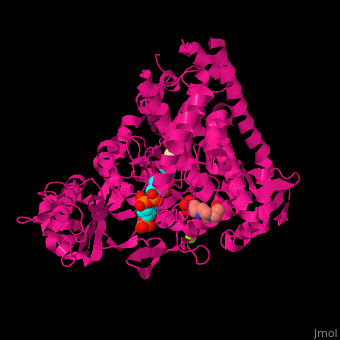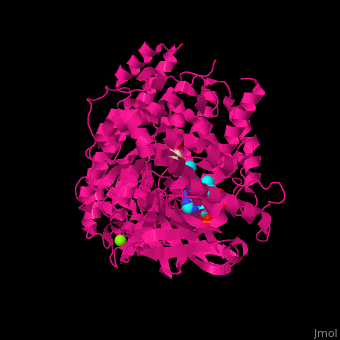
FunctionMalate synthase (MS) catalyzes the reversible conversion of acetyl-CoA, glyoxylate and water to (S)-malate and CoA. MS participates in pyruvate, glyoxylate and dicarboxylate metabolism. There are 2 isozymes of MS. MS G is formed during growth on glycolate[1] and MS A which metabolizes glyoxylate formed in the dissimilation of acetate. See also: Glyoxylate cycle Structural highlights. The ternary complex contains malate, acetyl-CoA and Mg+2 ion[2]. . Water molecules are shown as red spheres. . 3d structures of malate synthase
|
| ||||||||||
ReferencesReferences
- ↑ Anstrom DM, Kallio K, Remington SJ. Structure of the Escherichia coli malate synthase G:pyruvate:acetyl-coenzyme A abortive ternary complex at 1.95 A resolution. Protein Sci. 2003 Sep;12(9):1822-32. PMID:12930982
- ↑ Anstrom DM, Remington SJ. The product complex of M. tuberculosis malate synthase revisited. Protein Sci. 2006 Aug;15(8):2002-7. PMID:16877713 doi:15/8/2002