Ferguson Gal4 sandbox

| |||||||||
| 1d66, resolution 2.70Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
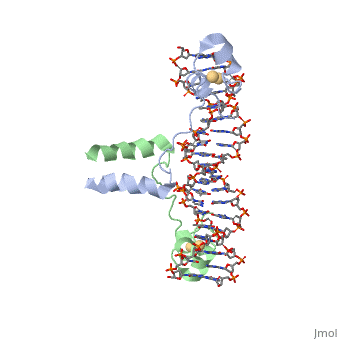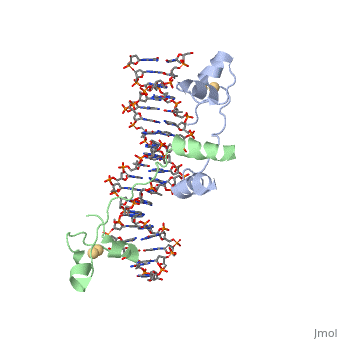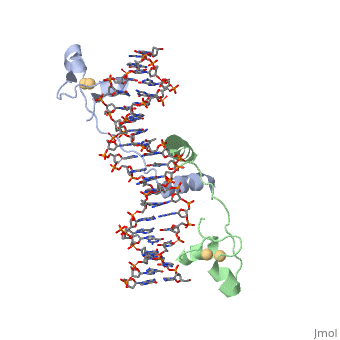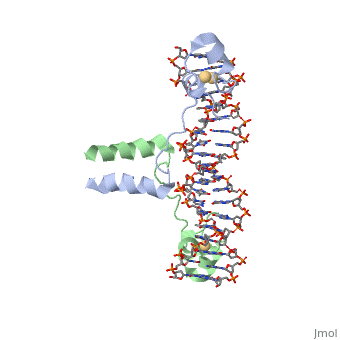
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEXDNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4.
About this StructureAbout this Structure
1d66 is a 4 chain structure with sequence from Saccharomyces cerevisiae. Full crystallographic information is available from OCA.
See AlsoSee Also
ReferenceReference
- ↑ Marmorstein R, Carey M, Ptashne M, Harrison SC. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122 doi:http://dx.doi.org/10.1038/356408a0