Dinoflagellate luciferase
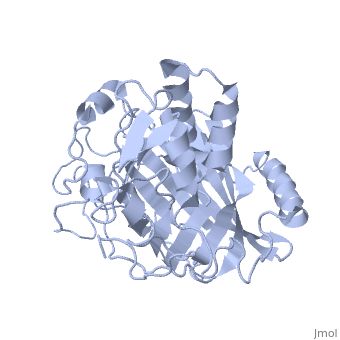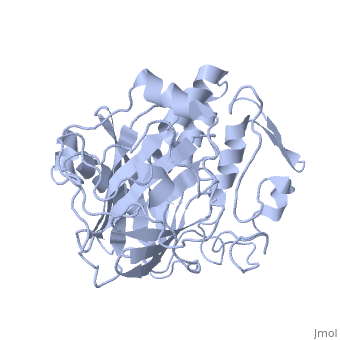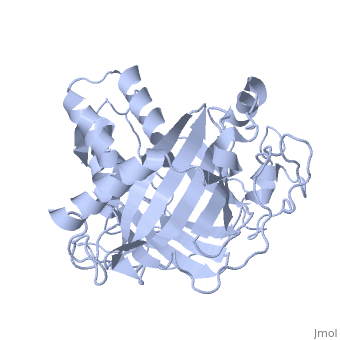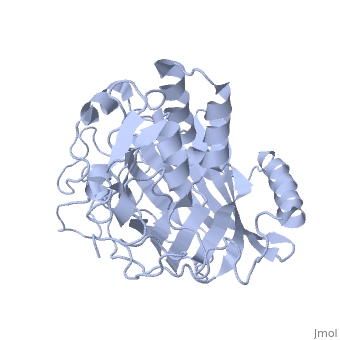
IntroductionLingulodinium polyedrum, a marine dinoflagellate often responsible for red tide, possesses a unique luciferase enyzme. When mechanically stimulated, the organism uses this enzyme to produce a blue light, likely for use in quorum sensing. Other luciferase enzymes typically produce green-yellow to red light. Also, while all luciferase enzymes produce light through oxidation of luciferin, the biochemical mechanism by which this is achieved varies. This means that while the result is the same, there is low similarity to bacterial or firefly luciferases. In L. polyedrum, the luciferase enzyme is a single polypeptide chain folded into 3 similar domains. Interestingly, all three domains appear to be distinct luciferase centres with their own catalytic activities [1] StructureComposed of residues 868-1218, domain 3 (D3) also consists of a 20aa C-terminal unresolved domain. Containing 7 α-helices and 16 β-strands, D3 is further organized into subdomains. The main portion of the enzyme appears to be a β-barrel structure composed of 10 anti-parallel strands connected via a Gly rich sequence to a 3 helix bundle. This bundle is stabilized by a hydrophobic core region as well as a multitude of H-bonding patterns[1]. The β-barrel structure actually has some homology with the human muscle fatty acid binding protein (m-FABP, pdb= 1hmt). Both are part of a "β-clam" subdomain family, responsible for binding of hydrophobic molecules. However, other known β-clam structures do not possess enzymatic activity[1].

Note the position of the tri-helix in front of the β-barrel opening, blocking substrate entry. Under a pH of 8, the protonation states of the four histidines are thought to drive a conformational change that opens and expands the β-barrel. Interestingly, the four histidines(H899, H909, H924 and H930) are conserved in another dinoflagellate, Pyrocystis lunula[2]. This seems to suggest a pH-dependant luciferase contol mechanism similiar to the proposed mechanism in L. polyedrum. While related at the primary structure level, the structure of P. lunula luciferase has yet to be solved, so further structural similarities cannot be easily determined. Luciferase ReactionTypically, luciferases produce light through a high energy complex with a luciferin cofactor, and Mg-ATP. The structure of luciferin is different from organism to organism, and in L. polyedrum, is a chlorophyll-derived open-tetrapyrrole. Below is the dinoflagellate luciferase reaction, showing the oxidation site.
 Image courtesy of L. Wayne Schultz. References
Content DonorsAll the initial portions of this page were created by James Jones and were moved because it deserved its own separate page distinct from the associated PDB entry. |
| ||||||||||
3D structures of luciferase3D structures of luciferase
See AlsoSee Also
- 1vpr is the structure used on this page.
- Colored & Bioluminescent Proteins
- PyMOL