2hue
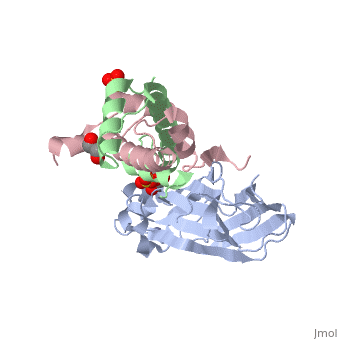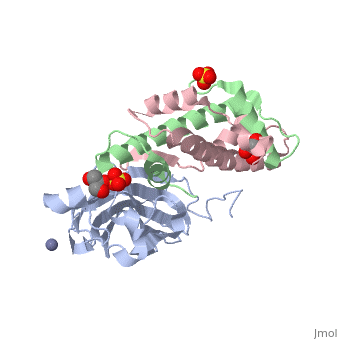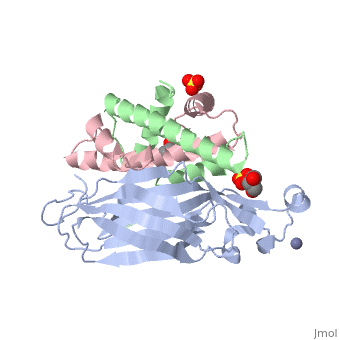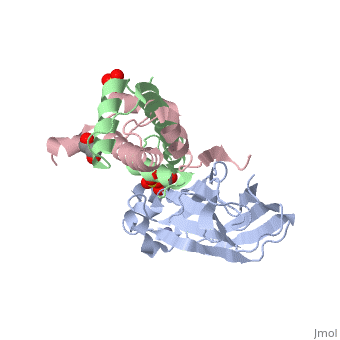
Structure of the H3-H4 chaperone Asf1 bound to histones H3 and H4
|
OverviewOverview
Anti-silencing function 1 (Asf1) is a highly conserved chaperone of, histones H3/H4 that assembles or disassembles chromatin during, transcription, replication, and repair. The structure of the globular, domain of Asf1 bound to H3/H4 determined by X-ray crystallography to a, resolution of 1.7 Angstroms shows how Asf1 binds the H3/H4 heterodimer, enveloping the C terminus of histone H3 and physically blocking formation, of the H3/H4 heterotetramer. Unexpectedly, the C terminus of histone H4, that forms a mini-beta sheet with histone H2A in the nucleosome undergoes, a major conformational change upon binding to Asf1 and adds a beta strand, to the Asf1 beta sheet sandwich. Interactions with both H3 and H4 were, required for Asf1 histone chaperone function in vivo and in vitro. The, Asf1-H3/H4 structure suggests a "strand-capture" mechanism whereby the H4, tail acts as a lever to facilitate chromatin disassembly/assembly that may, be used ubiquitously by histone chaperones.
About this StructureAbout this Structure
2HUE is a Protein complex structure of sequences from Saccharomyces cerevisiae and Xenopus laevis with SO4, ZN and GOL as ligands. Full crystallographic information is available from OCA.
ReferenceReference
Structural basis for the histone chaperone activity of Asf1., English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK, Cell. 2006 Nov 3;127(3):495-508. PMID:17081973
Page seeded by OCA on Wed Nov 21 11:59:31 2007