User:James D Watson/Structural Templates
Motifs In ProteinsMotifs In Proteins
The term "motif" when used in structural biology tends to refer to one of two cases:
- A particular amino-acid sequence that characterises a biochemical function
- A set of secondary structure elements that defines a functional or structural role
There are a great number of protein sequence motifs identified, many of which have well defined structural or functional roles. One such example of this is the so-called zinc finger motif which is readily identified from the following consensus sequence pattern (where "X" represents any amino acid):
Cys - X(2-4) - Cys - X(3) - Phe - X(5) - Leu - X(2) - His - X(3) - His
|
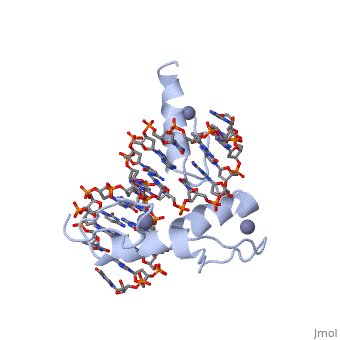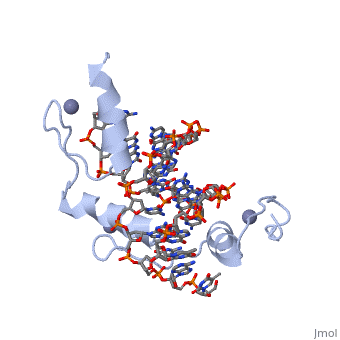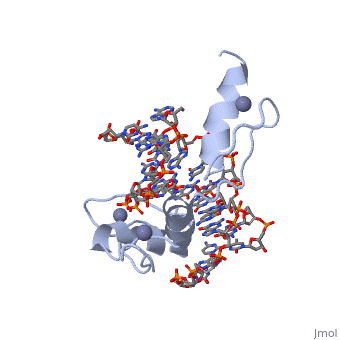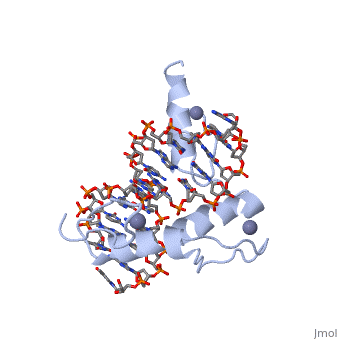
The example structure shown to is that of Zif268 protein-DNA complex from Mus musculus (PDB entry 1AAY). In this example (a C2H2 class zinc finger) the conserved and histidine residues form ligands to a zinc ion whose coordination is essential to stabilise the tertiary fold of the protein. The fold is important because it helps orientate the recogniton helices to bind to the DNA.
However, there are also a number of repeated patterns and functional motifs revealed when the protein structure is examined. This page aims to introduce some of the main types of motif illustrating them on protein structures from the PDB.
Secondary structure elementsSecondary structure elements
| |||||||||
| 5p21, resolution 1.35Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
The image to the right shows the crystal structure of the H-ras oncogene protein p21 complexed to a substrate analogue (GppNp).
About this StructureAbout this Structure
5P21 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
ReferenceReference
Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis., Pai EF, Krengel U, Petsko GA, Goody RS, Kabsch W, Wittinghofer A, EMBO J. 1990 Aug;9(8):2351-9.
Structural superpositionStructural superposition
The viewer below left shows the structural superposition of the triphosphate conformation of H-ras p21 (PDB entry 5p21 - coloured orange) with Gdp-bound human rab21 gtpase (PDB entry 1z0i - coloured blue), the structural superposition was made using the MSDfold(SSM)1 server at the EBI2. Note that the global fold of these two proteins is almost identical yet their sequence identity is only 29.6% (as determined using FASTA). The viewer below right shows the p-loops of both structures superposed. The ligands bound superpose particularly well and comparison of the two p-loops loops show significant structural similarity but also highlights the sequence differences between the two proteins.
|
|
This is a placeholderThis is a placeholder
This is a placeholder text to help you get started in placing a Jmol applet on your page. At any time, click "Show Preview" at the bottom of this page to see how it goes.
Replace the PDB id after the STRUCTURE_ and after PDB= to load and display another structure.
| |||||||||
| 3cin, resolution 1.70Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | TM1419, TM_1419 (Thermotoga maritima MSB8) | ||||||||
| Activity: | Inositol-3-phosphate synthase, with EC number 5.5.1.4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||