Prion protein
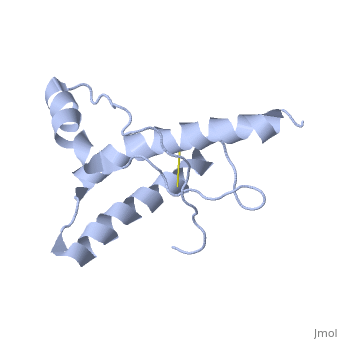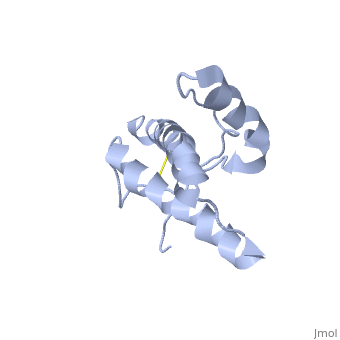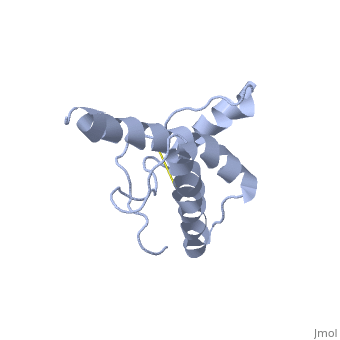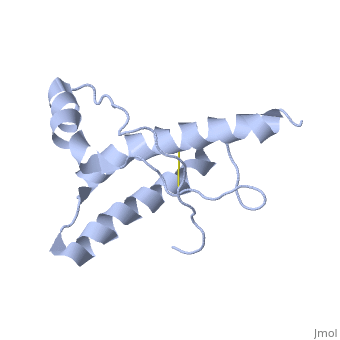
The prion protein (PrP) is a cell surface glycoprotein. The cellular isoform (PrPC) has a natively unstructured N-terminal region, and a predominantly α-helical C-terminal region from residues ~120-230. PrPC can undergo a structural conversion to a β-sheet rich conformation, termed PrPSc. Prion diseases such as Creutzfeldt Jakob disease (CJD) in people, and bovine spongiform encephalopathy (BSE) commonly known as "mad cow" disease, are characterterized by aggregates of PrPSc, which arise from autocatalytic refolding of PrPC in a template-dependent manner.
Structure of PrPCStructure of PrPC
| |||||||||
| 1hjm, 1 NMR models () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Related: | 1e1g, 1e1j, 1e1p, 1e1s, 1e1u, 1e1w, 1hjn | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
The structure is highly conserved amongst mammals...
The X-ray structure of sheep PrP was dimeric...
Models of PrPSc structureModels of PrPSc structure
There are a number of technical obstacles in determining the molecular structure of PrP(sup)Sc
Selected PrP structuresSelected PrP structures
All structures determined by NMR unless otherwise specified
Human PrPHuman PrP
- 1QLX HuPrP 23-230
- 1QM0 HuPrP 90-230
- 1QM2 HuPrP 121-230
- 1I4M HuPrP 119-226 (X-ray)
- 1E1J HuPrP,M166V 125-228 Average 125-228
- 1E1S HuPrP,S170N 125-228 Average 125-228
- 1E1W HuPrP,R220K 125-228 Average 125-228
- 1FKC HuPrP,E200K residues 90-231 (genetic prion disease)
- 1H0L HuPrP residues 121-230, with an additional disulphide bond analogous to the homolog Doppel