Sandbox108
Glutamine synthetase assignment by UMBC undergraduate studentsGlutamine synthetase assignment by UMBC undergraduate students
| |||||||||
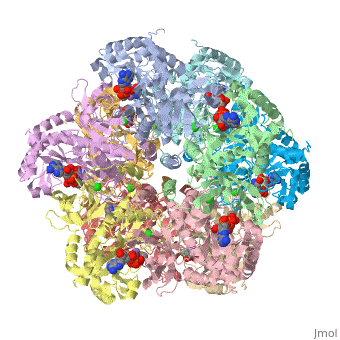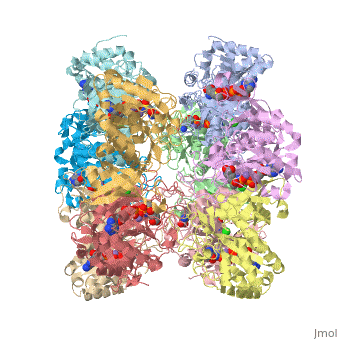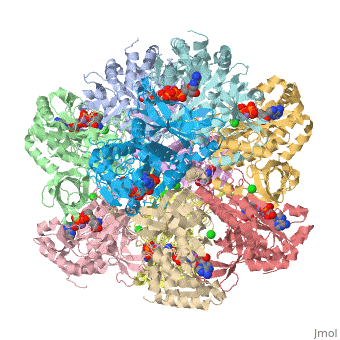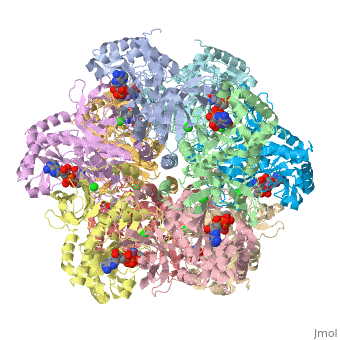
| 2qc8, resolution 2.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Gene: | GLUL, GLNS (Homo sapiens) | ||||||||
| Activity: | Glutamate--ammonia ligase, with EC number 6.3.1.2 | ||||||||
| Related: | 2ojw | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
OUTLINEOUTLINE
Tertiary Structure
Different groups of amino acids specify a variety of properties of their side chains. In general, there are five categories; non-polar and aliphatic R groups, aromatic R groups, polar and uncharged R groups, positively charged R groups, and negatively charged R groups.
Glutamine is within uncharged polar <show the uncharged polar groups wiki text>. Usually, uncharged polar groups are classified as hydrophilic <show the hydrophilic wiki text> that is found on the outside of proteins. Also, amino acids with the character of acidic or basic side chains are polar, showing on the outside of molecules <show the polar wiki text>. For glutamine, its side chain is uncharged and formed by replacing the hydroxyl of glutamic acid with an amine functional group. [1] In the other hand, glutamine has no side chain on non-polar group, however the side chain on non-polar groups of the proteins usually tends to be hydrophobic <show the hydrophobic of cysteine wiki text> and to cluster together on the inside.[2]
Tertiary structure of protein is characterized by the “global” folding of a polypeptide chain. [3] Hydrophobic interaction is a major driving force determining the tertiary structure of the proteins. [4] The reason why hydrophobic interaction is important is because of relationship with the hydrogen bonding. The peptide backbone is hydrophilic, but in the middle of proteins is mostly in a hydrophobic circumstance. So, in order to reduce the hydrophilicity, to maximize the hydrogen bonding, the α-helix <show α-helix wiki text> and the β-sheet <show the β-sheet wiki text> can break down the C=O and N-H groups in the peptide bonds so that the hydrogen bonds are maximum. [5] Also, all polar and hydrophilic side chains interact with H-bonds. Hydrogen bonding <show the H.B wiki text> is crucial in stabilizing the tertiary structure. [6] On the other hand, disulfide bonds <show the disulfide bonds of cysteine wiki text> between cysteine residues stabilize the tertiary structure. [7]