Hydrogen bonds
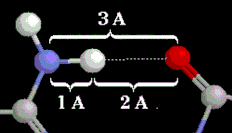 | |
Elements C, H, N, O. A hydrogen bond between a nitrogen donor and an oxygen acceptor. Distances shown are typical for those found in proteins. |
Hydrogen bonds occur when a "donor" atom donates its covalently bonded hydrogen atom to an electronegative "acceptor" atom. The oxygen in -OH (e.g. Ser, Thr, Tyr), HOH, and the nitrogen in -NH3+ (as in Lys, Arg) or -NH- (as in the main chain peptide bond, Trp, His, Arg, nucleotide bases) are typical donors. The lone electron pairs on these same donors can serve as hbond acceptor sites. So can those on carbonyl oxygens =O (as in the main chain) or nitrogens with three covalent bonds =N- (as in His, Trp, or nucleotide bases). Lacking hydrogens, these latter cannot serve as donors.
Jeffrey[1] categorizes hbonds with donor-acceptor distances of 2.2-2.5 Å as "strong, mostly covalent", 2.5-3.2 Å as "moderate, mostly electrostatic", 3.2-4.0 Å as "weak, electrostatic" (page 12). Energies are given as 40-14, 15-4, and <4 kcal/mol respectively. Most hbonds in proteins are in the moderate category, strong hbonds requiring moieties or conditions that are rare within proteins. The hydrogen atoms in moderate hbonds often do not lie on the straight line connecting the donor to acceptor, so donor-acceptor distance slightly underestimates the length of the hbond (Jeffrey[1], p. 14). The mean donor-acceptor distances in protein secondary structure elements are close to 3.0 Å, as are those between bases in Watson-Crick pairing (Jeffrey[1], pp. 191, 200). Since many PDB files lack hydrogen atoms, the presence of an energetically significant hydrogen bond can be inferred when a probable donor and acceptor are within 3.5 Å of each other. PE's DISPLAY Contacts defines "likely noncovalently bonded" oxygens and nitrogens (shown as balls) as those within 3.5 Å of other oxygens and nitrogens.
Content AttributionContent Attribution
The text initially provided on this page was adapted by Eric Martz from the hydrogen bonds entry that he wrote several years earlier for the glossary in ProteinExplorer.Org.