1jqn
|
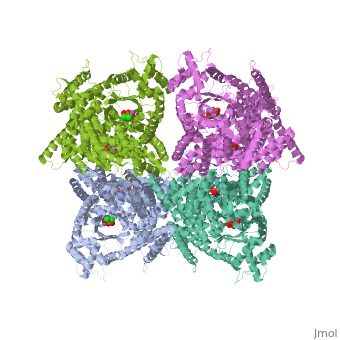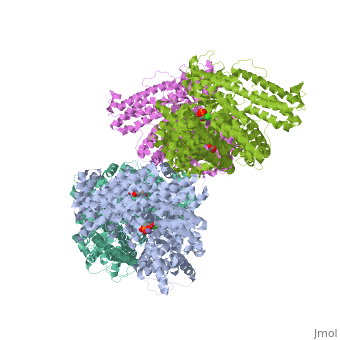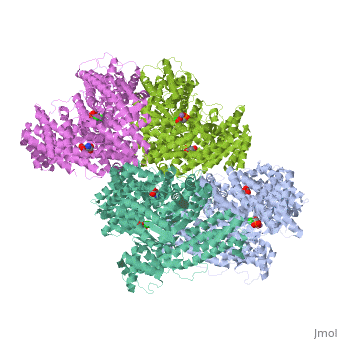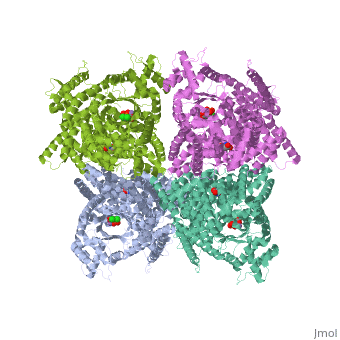
Crystal structure of E.coli phosphoenolpyruvate carboxylase in complex with Mn2+ and DCDP
OverviewOverview
Phosphoenolpyruvate carboxylase (PEPC) catalyzes the first step in the, fixation of atmospheric CO(2) during C(4) photosynthesis. The crystal, structure of C(4) form maize PEPC (ZmPEPC), the first structure of the, plant PEPCs, has been determined at 3.0 A resolution. The structure, includes a sulfate ion at the plausible binding site of an allosteric, activator, glucose 6-phosphate. The crystal structure of E. coli PEPC, (EcPEPC) complexed with Mn(2+), phosphoenolpyruvate analog, (3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate), and an, allosteric inhibitor, aspartate, has also been determined at 2.35 A, resolution. Dynamic movements were found in the ZmPEPC structure, compared, with the EcPEPC structure, around two loops near the active site. On the, basis of these molecular structures, the mechanisms for the carboxylation, reaction and for the allosteric regulation of PEPC are proposed.
About this StructureAbout this Structure
1JQN is a Single protein structure of sequence from Escherichia coli with MN, ASP and DCO as ligands. Active as Phosphoenolpyruvate carboxylase, with EC number 4.1.1.31 Full crystallographic information is available from OCA.
ReferenceReference
Crystal structures of C4 form maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases., Matsumura H, Xie Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y, Structure. 2002 Dec;10(12):1721-30. PMID:12467579
Page seeded by OCA on Tue Nov 20 18:29:19 2007