1aay
ZIF268 ZINC FINGER-DNA COMPLEX
|
OverviewOverview
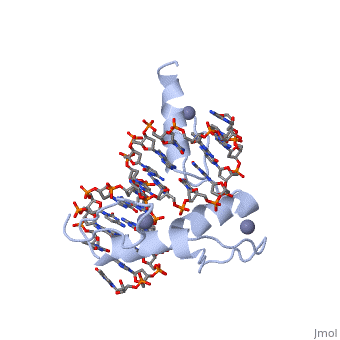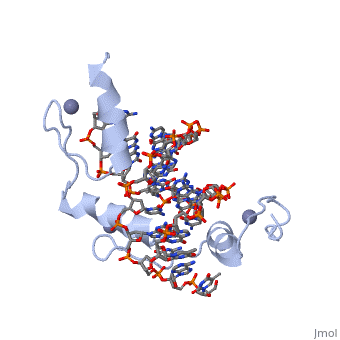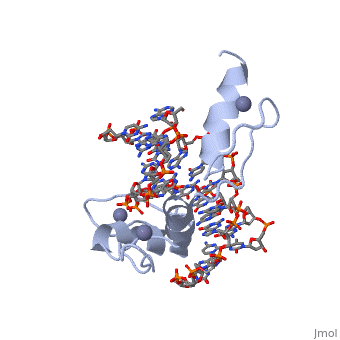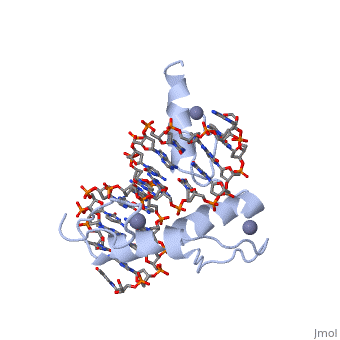
BACKGROUND: Zinc fingers of the Cys2 His2 class recognize a wide variety, of different DNA sequences and are one of the most abundant DNA-binding, motifs found in eukaryotes. The previously determined 2.1 A structure of a, complex containing the three zinc fingers from Zif268 has served as a, basis for many modeling and design studies, and Zif268 has proved to be a, very useful model system for studying how TFIIIA-like zinc fingers, recognize DNA. RESULTS: We have refined the structure of the Zif268, protein-DNA complex at 1.6 A resolution. Our structure confirms all the, basic features of the previous model and allows us to focus on some, critical details at the protein-DNA interface. In particular, our refined, structure helps explain the roles of several acidic residues located in, the recognition helices and shows that the zinc fingers make a number of, water-mediated contacts with bases and phosphates. Modeling studies, suggest that the distinctive DNA conformation observed in the Zif268-DNA, complex is correlated with finger-finger interactions and the length of, the linkers between adjacent fingers. Circular dichroism studies indicate, that at least some of the features of this distinctive DNA conformation, are induced upon complex formation. CONCLUSIONS: Our 1.6 A structure, should provide an excellent framework for analyzing the effects of Zif268, mutations, for modeling related zinc finger-DNA complexes, and for, designing and selecting Zif268 variants that will recognize other DNA, sites.
About this StructureAbout this Structure
1AAY is a Single protein structure of sequence from Mus musculus with ZN as ligand. Full crystallographic information is available from OCA.
ReferenceReference
Zif268 protein-DNA complex refined at 1.6 A: a model system for understanding zinc finger-DNA interactions., Elrod-Erickson M, Rould MA, Nekludova L, Pabo CO, Structure. 1996 Oct 15;4(10):1171-80. PMID:8939742
Page seeded by OCA on Tue Nov 20 10:43:35 2007