2pl1
| |||||||||
| 2pl1, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Non-Standard Residues: | |||||||||
| Gene: | phoP (Escherichia coli) | ||||||||
| Related: | 2pkx | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
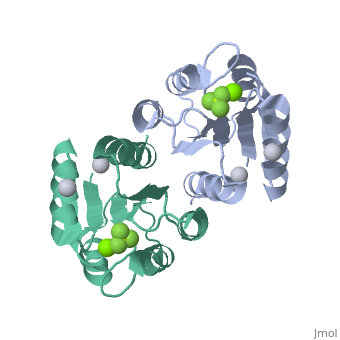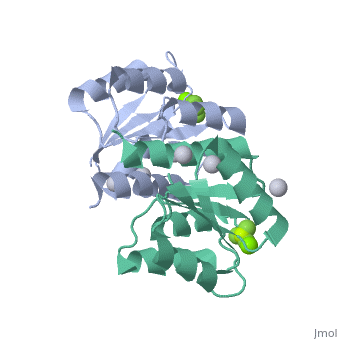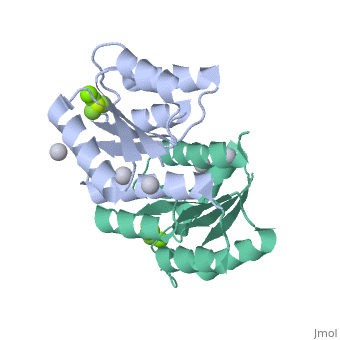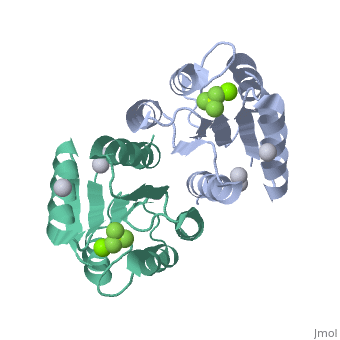
Berrylium Fluoride activated receiver domain of E.coli PhoP
OverviewOverview
The response regulator PhoP is part of the PhoQ/PhoP two-component system involved in responses to depletion of extracellular Mg(2+). Here, we report the crystal structures of the receiver domain of Escherichia coli PhoP determined in the absence and presence of the phosphoryl analog beryllofluoride. In the presence of beryllofluoride, the active receiver domain forms a twofold symmetric dimer similar to that seen in structures of other regulatory domains from the OmpR/PhoB family, providing further evidence that members of this family utilize a common mode of dimerization in the active state. In the absence of activating agents, the PhoP receiver domain crystallizes with a similar structure, consistent with the previous observation that high concentrations can promote an active state of PhoP independent of phosphorylation.
About this StructureAbout this Structure
2PL1 is a Single protein structure of sequence from Escherichia coli. This structure supersedes the now removed PDB entry 2eub. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride., Bachhawat P, Stock AM, J Bacteriol. 2007 Aug;189(16):5987-95. Epub 2007 Jun 1. PMID:17545283 Page seeded by OCA on Sun May 4 13:20:05 2008