1io1
CRYSTAL STRUCTURE OF F41 FRAGMENT OF FLAGELLINCRYSTAL STRUCTURE OF F41 FRAGMENT OF FLAGELLIN
Structural highlights
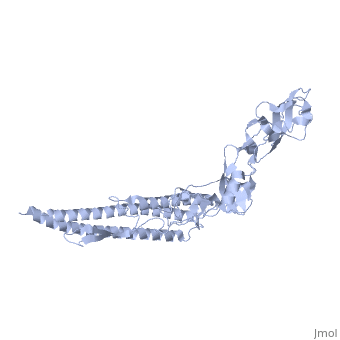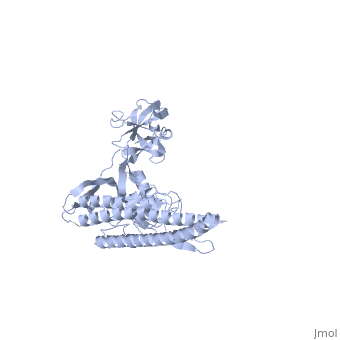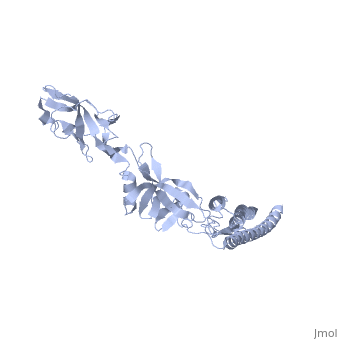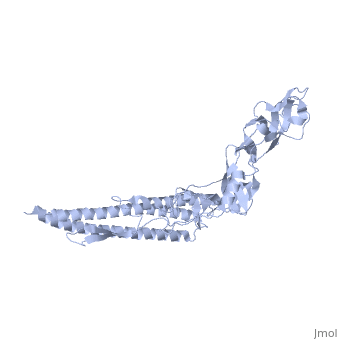
FunctionFLIC_SALTY Flagellin is the subunit protein which polymerizes to form the filaments of bacterial flagella. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe bacterial flagellar filament is a helical propeller constructed from 11 protofilaments of a single protein, flagellin. The filament switches between left- and right-handed supercoiled forms when bacteria switch their swimming mode between running and tumbling. Supercoiling is produced by two different packing interactions of flagellin called L and R. In switching from L to R, the intersubunit distance ( approximately 52 A) along the protofilament decreases by 0.8 A. Changes in the number of L and R protofilaments govern supercoiling of the filament. Here we report the 2.0 A resolution crystal structure of a Salmonella flagellin fragment of relative molecular mass 41,300. The crystal contains pairs of antiparallel straight protofilaments with the R-type repeat. By simulated extension of the protofilament model, we have identified possible switch regions responsible for the bi-stable mechanical switch that generates the 0.8 A difference in repeat distance. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling.,Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K Nature. 2001 Mar 15;410(6826):331-7. PMID:11268201[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||