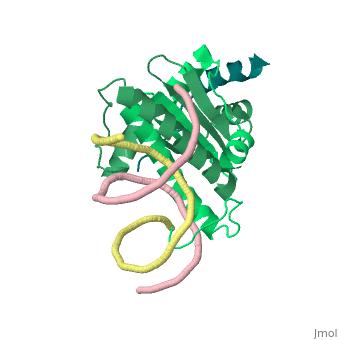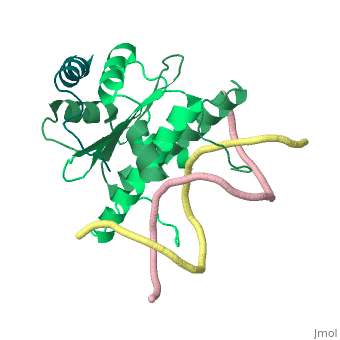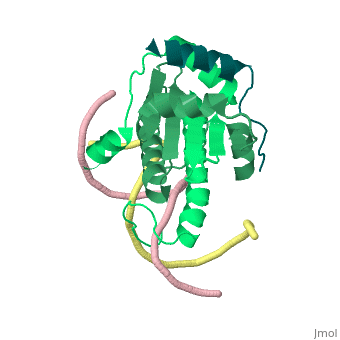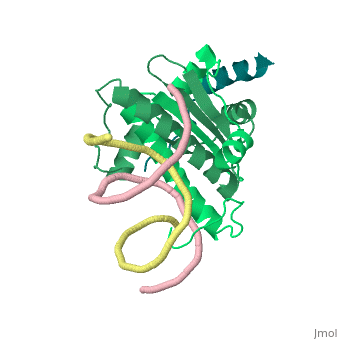1tqe
Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2
Structural highlights
FunctionMEF2B_HUMAN Transcriptional activator which binds specifically to the MEF2 element, 5'-YTA[AT](4)TAR-3', found in numerous muscle-specific genes. Activates transcription via this element. May be involved in muscle-specific and/or growth factor-related transcription. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedClass II histone deacetylases (HDACs) bind myocyte enhancer factor-2 (MEF2) and repress specific gene expression in a calcium-dependent manner. Despite their significant physiological functions in muscle, immune and neuronal cells, the mechanism of recruitment of class II HDACs by MEF2 is not well understood. Here, we have characterized the complex between the MEF2-binding motif of class II HDACs and the MADS-box/MEF2S domain of MEF2B by structural and biochemical methods. The crystal structure of a HDAC9/MEF2/DNA complex reveals that HDAC9 binds to a hydrophobic groove of the MEF2 dimer. The overall binding mode is similar to that seen in the Cabin1/MEF2/DNA complex. The detailed binding interactions at the HDAC9/MEF2 interface, however, show marked differences from those at the Cabin1/MEF2 interface. Our studies suggest a general mechanism by which class II HDACs and possibly other transcriptional co-regulators are recruited by MEF2. On the other hand, the differential binding between MEF2 and its various partners may confer specific regulatory and functional properties to MEF2 in distinct cellular processes. Such specificity provides a basis for selectively disrupting a particular MEF2/co-regulator complex by mutations or small molecules. Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2.,Han A, He J, Wu Y, Liu JO, Chen L J Mol Biol. 2005 Jan 7;345(1):91-102. PMID:15567413[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||