Hemoglobin
|
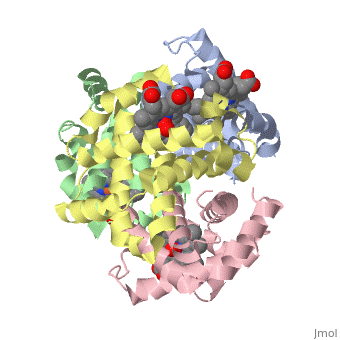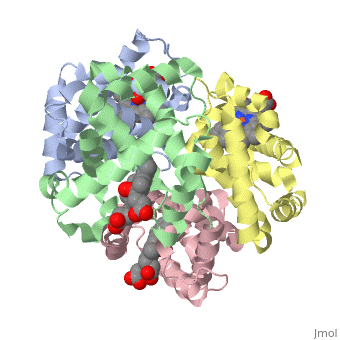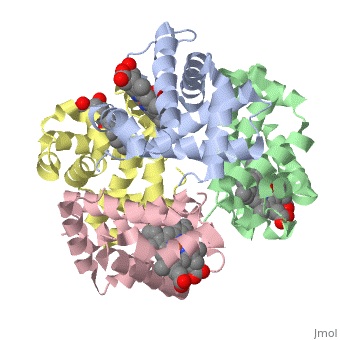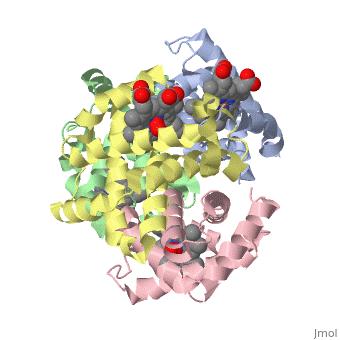
Hemoglobin is an oxygen-transport protein. Hemoglobin is an allosteric protein. It is a tetramer composed of two types of subunits designated α and β, whose stoichiometry is . The of hemoglobin sit roughly at the corners of a tetrahedron, facing each other across a at the center of the molecule. Each of the subunits prosthetic group. The give hemoglobin its red color.
Each individual molecule contains one atom. In the lungs, where oxygen is abundant, an binds to the ferrous iron atom of the heme molecule and is later released in tissues needing oxygen. The heme group binds oxygen while still attached to the . The spacefill view of the hemoglobin polypeptide subunit with an oxygenated heme group shows how the within the polypeptide.
is facilitated by a histidine nitrogen that binds to the iron. A second histidine is near the bound oxygen. The "arms" (propanoate groups) of heme face are hydrophilic and face the surface of the protein while the hydrophobic portions of the heme are buried among the hydrophobic amino acids of the protein. We can also view the anchored heme with the hemoglobin polypeptide subunit shown in a representation.
Secondary structureSecondary structure
Most of the amino acids in hemoglobin form , connected by short non-helical segments. Hemoglobin has no beta strands and no disulfide bonds. A rainbow coloring scheme from N-terminus (blue) to C-terminus (red) helps to discern the . This is at the protein-water interface.
Hydrophobicity, polarity and chargeHydrophobicity, polarity and charge
This view of the shows charged amino acids (lime green), polar amino acids (dark green), hydrophobic amino acids (grey), heme (tan), and water oxygens (light blue). Charged and polar amino acids are common on the surface, where they form hydrogen bonds with water. Most water is not shown. Protein structures tend to fold in such a way so that their hydrophobic residues are buried and their hydrophilic residues are on the surface. A vertical slice through the molecule near the front surface, shows . As we move the slice plane into the molecule, the center is hydrophobic while the surfaces have many polar and charged residues. We can move the slab , and . Near the rear surface, hydrophilic residues are .