Estrogens
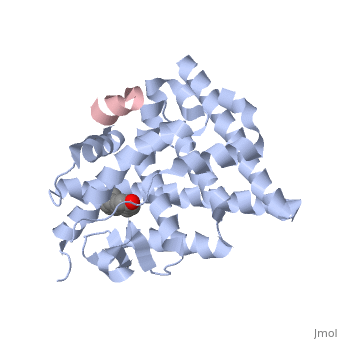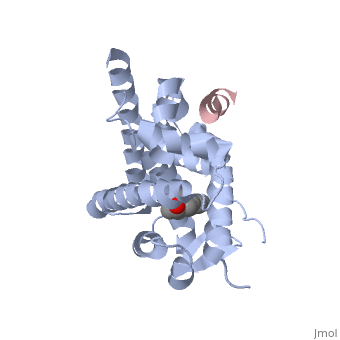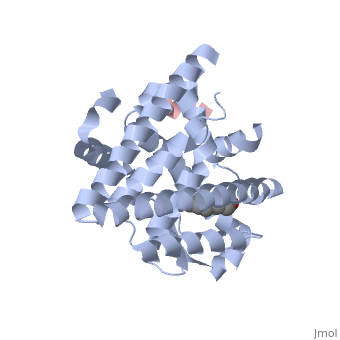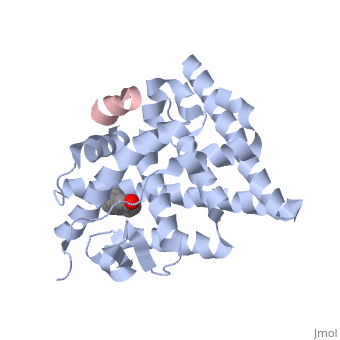
Estrogensof estrogen receptor α complexed with raloxifene and a corepressor peptide (morph was taken from Gallery of Morphs of the Yale Morph Server). Structure of estradiol metal chelate and estrogen receptor complex: The basis for designing a new class of SERMs[1]. Selective estrogen receptor modulators, such as estradiol 17-derived metal complexes, have been synthesized as targeted probes for the diagnosis and treatment of breast cancer. The detailed 3D structure of bound with a novel at 2.6Å resolution was reported (2yat). The residues with EPTA-Eu. The hydrogen bonds are shown as white dashed lines. of this structure with the structure of native ligand 17β-estradiol (E2) in the complex of E2/ERα-LBD complex (1ere) reveals that the . The made by additional estrogen receptor residues (e.g. Glu419 of H7 and Glu339 of H3, this depends on subunit), may work together with the E2 17β hydroxyl-His524 hydrogen bond and tighten the neck of the LBP upon binding of the endogenous ligand E2. 4-Hydroxytamoxifen (OHT) is an other selective estrogen receptor modulator. of EPTA-Eu/ERα-LBD complex on OHT/ERα-LBD complex (3ert) shows that there is similar network of hydrogen bonds in both complexes, except for His524 which does not form hydrogen bond with OHT in the OHT/ERα-LBD complex. E2/ERα-LBD (1ere), OHT/ERα-LBD (3ert) and EPTA-Eu/ERα-LBD shows that they overlap well in the majority portions of the domain, but differ significantly in the region of the 'omega loop'. They display different synergistic reciprocating movements, depending on the specific nature of the ligand bound. The structure of estrogen receptor complexed with EPTA-Eu provides important information pertinent to the design of novel functional ER targeted probes for clinical applications. ER is a modular protein composed of a ligand binding domain, a DNA binding domain and a transactivation domain. ER is a DNA-binding transcription factor. . The DNA binding domain can be clearly observed in this scene; the highlighted yellow helix in close proximity to the DNA is part of the DNA binding domain. The blue beta sheet close to the yellow DNA binding alpha helix is also part of the DNA binding domain. The transactivation domain forms an alpha helix which is colored in purple. The transactivation domain activates RNA polymerase when the receptor binds to DNA. The ligand binding domain may be observed here with the following scene. . The ligand ferutinine (highlighted in pink) is bound by the ligand binding domain, composed of the blue colored alpha helices immediately surrounding the purple ligand. Another view of the ligand binding domain is shown here, with estradiol bound. . ER is functional as a ligand-dependent transcription factor. ER responds to both agonist and antagonist ligands and can associate with the nuclear matrix. Differences in the structure of the receptor are observed depending on what ligand ER has bound (if any). Through comparisons of ER bound to agonist and antagonist ligands, some structural components may be highlighted. The specific conformation of this tight loop of alpha helices and beta sheets around the ligand shows a complex capable of activating ER's transcription loci. This complex allows for the activation signal that will stimulate normal growth. Normal growth is stimulated when an agonist bound ER binds DNA. This occurs with the assistance of chaperon proteins. These chaperons are capable of recognizing estrogen receptor ligand complexes. When ER has bound a ligand chaperons facilitate the trans-location of the complex to the nucleus. Eventually the chaperon ligand ER complex will reach specific euchromatin, at which point the chaperons facilitate the ligand ER complex to changes conformation. This conformation will facilitate the estrogen receptor to bind the DNA major groove at specific palindromic sequences. Estradiol is a normal ligand for ER and allows for binding in the major groove of DNA. If the ligand is an antagonist the transcription factor function of estrogen receptor becomes hindered. The conformation of ER bound to the partial agonist genistein has a loop which is not as tight around the ligand as those found on ER with a complete agonist ligand. The ligands themselves take up different amounts of space and have varying interactions within ER. This slight difference effects the ability of the chaperon to be able to bind the receptor ligand complex to the major groove of DNA. There is a noticeable difference in the size of the pure agonist vs partial agonist scenes. Specifically, look at the width of the agonist compared to the partial agonist. Similar differences may be observed between ER which has bound the partial agonist and complete antagonist ligands. The most drastic difference is noticeable between agonist and antagonist ligands. Compare the agonist scene to the . Special attention should be given to the bottom right alpha helices and beta sheets that are pushed out more in the antagonist compared to the agonist bound ER.
|
| ||||||||||
See also:
ReferencesReferences
- ↑ Li MJ, Greenblatt HM, Dym O, Albeck S, Pais A, Gunanathan C, Milstein D, Degani H, Sussman JL. Structure of estradiol metal chelate and estrogen receptor complex: The basis for designing a new class of selective estrogen receptor modulators. J Med Chem. 2011 Apr 7. PMID:21473635 doi:10.1021/jm200192y