2a07
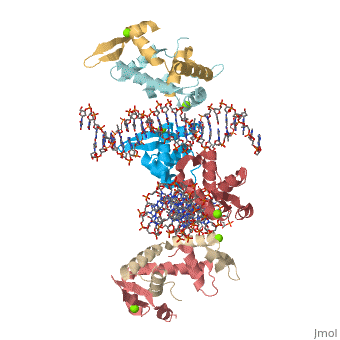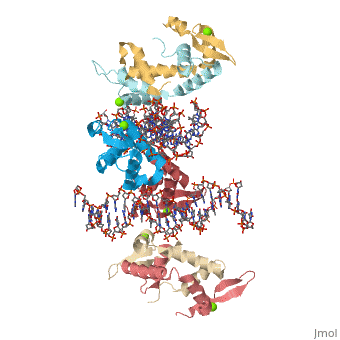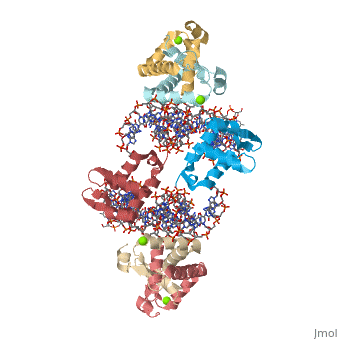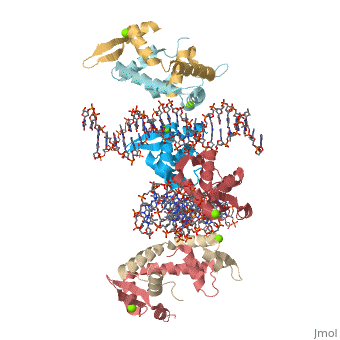
Crystal Structure of Foxp2 bound Specifically to DNA.Crystal Structure of Foxp2 bound Specifically to DNA.
Structural highlights
Disease[FOXP2_HUMAN] Defects in FOXP2 are the cause of speech-language disorder 1 (SPCH1) [MIM:602081]; also known as autosomal dominant speech and language disorder with orofacial dyspraxia. Affected individuals have a severe impairment in the selection and sequencing of fine orofacial movements, which are necessary for articulation. They also show deficits in several facets of language processing (such as the ability to break up words into their constituent phonemes) and grammatical skills.[1] Note=A chromosomal aberration involving FOXP2 is a cause of severe speech and language impairment. Translocation t(5;7)(q22;q31.2). Function[FOXP2_HUMAN] Transcriptional repressor that may play a role in the specification and differentiation of lung epithelium. May also play a role in developing neural, gastrointestinal and cardiovascular tissues. Can act with CTBP1 to synergistically repress transcription but CTPBP1 is not essential. Involved in neural mechanisms mediating the development of speech and language. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedFOXP (FOXP1-4) is a newly defined subfamily of the forkhead box (FOX) transcription factors. A mutation in the FOXP2 forkhead domain cosegregates with a severe speech disorder, whereas several mutations in the FOXP3 forkhead domain are linked to the IPEX syndrome in human and a similar autoimmune phenotype in mice. Here we report a 1.9 A crystal structure of the forkhead domain of human FOXP2 bound to DNA. This structure allows us to revise the previously proposed DNA recognition mechanism and provide a unifying model of DNA binding for the FOX family of proteins. Our studies also reveal that the FOXP2 forkhead domain can form a domain-swapped dimer, made possible by a strategic substitution of a highly conserved proline in conventional FOX proteins with alanine in the P subfamily. Disease-causing mutations in FOXP2 and FOXP3 map either to the DNA binding surface or the domain-swapping dimer interface, functionally corroborating the crystal structure. Structure of the forkhead domain of FOXP2 bound to DNA.,Stroud JC, Wu Y, Bates DL, Han A, Nowick K, Paabo S, Tong H, Chen L Structure. 2006 Jan;14(1):159-66. PMID:16407075[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||