Induced fit
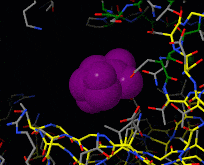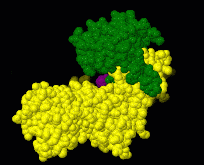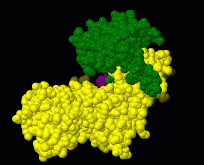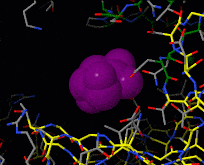
Induced fit describes a conformational change in a protein when it binds a ligand, in contrast to a lock-and-key model of ligand binding. A classic example of induced fit is binding of glucose to hexokinase, depicted in a morph between 3o8m and 3o80 in the picture at right.
History of the conceptHistory of the concept
Induced fit was suggested by Koshland in 1958 [1], providing an alternative to the lock-and-key binding model that Emil Fischer proposed in 1899 [2].
Interactive examplesInteractive examples
This is a morph of the (be patient when loading this). The first and the last frame of the animation are models based on crystallographic data while the intermediate states are somewhat arbitrary, but chosen to show a smooth transition between conformations.
|
| ||||||||||
See AlsoSee Also
- A morph of induced fit when Tamiflu binds to Neuraminidase.