Hormone sensitive lipase
This page, as it appeared on May 3, 2014, was featured in this article in the journal Biochemistry and Molecular Biology Education.
Hormone-sensitive lipases (HSL) represent a class of esterases within the α/β hydrolase family. Like other lipases, HSL catalyzes the cleavage of ester bonds, releasing fatty acid molecules. The activation and mobilization of hormone-sensitive lipase can be triggered by various catecholamines and inhibited by insulin.[1][2] Catecholamines, such as epinephrine, are rapidly spread throughout the body during times of energy mobilization, like in the fight or flight response. Conversely, insulin triggers glucose uptake, requiring the storage of energy, opposing HSL's function. HSL is clinically relevant, because the mobilization of or inability to mobilize fats in cells is directly related to fat accumulation seen in artherosclerosisand obesity.[3] Such diseases are characterized by an accumulation of fats and researchers are investigating whether HSL's activity plays a role or not.[2] Investigation of HSL's structure and function could provide a better clinical understanding of these diseases.[3]
Activation of HSLActivation of HSL
Briefly, HSL is stimulated by the binding of catecholamines to β-adrenergic receptors. β-adrenergic receptors coupled with adenylate cyclase (AC) then stimulate G-proteins to increase the levels of cystolic cAMP. Elevated levels of cAMP leads to an activation protein kinase A (PKA) leading to phosphorylation of serine residues on HSL activating and translocating HSL to lipid droplets for lipolysis. Conversely, insulin signaling decreases cystolic cAMP levels, resulting in a decreased HSL mobilization.[1]
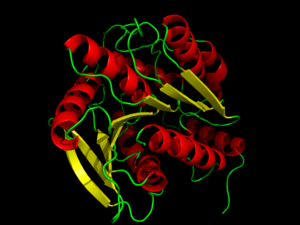
Structure of hormone-sensitive lipaseStructure of hormone-sensitive lipase
are generally well-conserved across domains, including prokaryotes, showing 29, 26, and 22% residue overlap in Alicyclobacillus acidocaldarius, Archaeoglobus fulgidus, and Bacillus subtilis, respectively.[4] HSL is composed of two main structural domains, consisting of a slightly variable N-terminus (shown in blue in the ) that is thought to contribute to numerous factors including activity, specificity, regioselectivity, thermophilicity, and thermostability.[4] Research speculates that the N-terminal domain, consisting of about 300 residues, mediates protein-protein interactions, and possibly subsequent lipid binding.[3] The second domain of HSL is the C-terminal catalytic domain (colors other than blue), which contains serine residue phosphorylation sites as well as the catalytic triad, viewed with ligand β-mercaptoethanol, a charge relay network that is characteristic of many hydrolases, such as chymotrypsin.[3] With respect to sequence conservation across species, it has been shown that the catalytic domain, including the triad, is conserved across domains, but the domain containing the N-terminus shows little conservation.[4] HSL has a that is approximately 16Å deep. Kinetic studies with substrates of varying lengths suggest that HSL primarily hydrolyzes shorter chained molecules.[4] Catalytic triadThe catalytic triad with β-mercaptoethanol toward the middle of HSL. The catalytic triad is composed of residues . The Ser157 residue sits at a site deemed the "nucleophilic elbow," that models an approximate torsion of Φ = 60° and Ψ =-120°.[4] This nucleophilic elbow is stabilized by a hydrogen bond between the proximal nitrogen and oxygen atoms of His281 and Glu251, respectively. This model also shows the strong nucleophilic character of Ser157, portraying the interaction and subsequent covalent bonding (not shown) to . Return to default view, .[3] Inhibition of hormone-sensitive lipaseHormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride (PMSF) covalently bound to the . PMSF is a general, covalent inhibitor of serine hydrolases, which has been co-crystallized bound to hormone-sensitive lipases. The experiments performed to test this inhibition used different lipases obtained from Bacillus coagulans as well as lipases from different areas of the human body.[5] PMSF inhibits hydrolase by binding to the catalytic serine residue of the serine protease active site which disrupts the nucleophilic activity of the catalytic serine. The sulfur of PMSF binds to the oxygen of the hydroxyl group on the serine residue to form this covalent bond. This inhibitor will only bind to the active site of the catalytic serine because of its participation in the charge relay of the catalytic triad. This hyper activity, indicated by increased temperature (shown in red) around the active site , allows the sulfonyl group of PMSF to covalently bind to the catalytic serine residue to disrupt its activity.[6] Because of this catalytic serine residue specificity, PMSF does not inhibit all kinds of lipases, such as pancreatic lipase and lipolase.Cite error: Closing Increased activity of HSL is also linked to disorders such as atherosclerosis, obesity, and type 2 diabetes. High concentration of free fatty acids (FFA) in skeletal muscles has been reported in many cases of obesity and type 2 diabetes. Increased inhibition of HSL through synthetic inhibitors could be a possible route for decreasing FFA concentration.[7]
|
| ||||||||||
Proteopedia PagesProteopedia Pages
ReferencesReferences
- ↑ 1.0 1.1 Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. PMID:14641008 doi:http://dx.doi.org/10.1042/
- ↑ 2.0 2.1 Ray H, Beylot M, Arner P, Larrouy D, Langin D, Holm C, Large V. The presence of a catalytically inactive form of hormone-sensitive lipase is associated with decreased lipolysis in abdominal subcutaneous adipose tissue of obese subjects. Diabetes. 2003 Jun;52(6):1417-22. PMID:12765952
- ↑ 3.0 3.1 3.2 3.3 3.4 Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004 Apr 1;379(Pt 1):11-22. PMID:14725507 doi:http://dx.doi.org/10.1042/BJ20031811
- ↑ 4.0 4.1 4.2 4.3 4.4 Nam KH, Kim MY, Kim SJ, Priyadarshi A, Kwon ST, Koo BS, Yoon SH, Hwang KY. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins. 2009 Mar;74(4):1036-40. PMID:19089974 doi:http://dx.doi.org/10.1002/prot.22313
- ↑ Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. Indian J Biochem Biophys. 2005 Aug;42(4):233-7. PMID:23923547
- ↑ 6.0 6.1 Nam KH, Kim SJ, Priyadarshi A, Kim HS, Hwang KY. The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads. Biochem Biophys Res Commun. 2009 Nov 13;389(2):247-50. Epub 2009 Aug 26. PMID:19715665 doi:10.1016/j.bbrc.2009.08.123
- ↑ Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002 Oct;43(10):1585-94. PMID:12364542
Student ContributorsStudent Contributors
Nathan Holt
Derek O'Connor