FunctionTGF-β receptors (Transforming Growth Factor) (TGFBR) are serine/threonine kinase receptors. They are involved in paracrine signaling and are found in many types of tissue. TGF-β ligands include bone morphogenetic proteins, growth and initiation factors, anti-Mullerian hormone, activin, nodal TGF-β[1]. There are 3 types of TGFBR:
- TGFBR I forms heteromeric complex with TGFBR II when it is bound to TGF-β. The complex transduces the TGF-β signal from the cell surface to the cytoplasm by phosphorylating proteins which regulate the transcription of genes related to cell proliferation. TGFBR I has high affinity for TGF-β1 and low affinity for TGF-β2.
- TGFBR II is a tumor suppressor transmembrane protein. TGFBR II has high affinity for TGF-β1 and low affinity for TGF-β2.
- TGFBR III is a cell-surface chondroitin sulfate / heparin sulfate proteoglycan. It acts as a reservoir of ligand for TGFBRs. TGFBR III has high affinity for TGF-β1, TGF-β2 and TGF-β1.2.
DiseaseOver-expression of TGF causes kidney disease, diabetes and renal disease. Mutations in TGFBR II cause various types of tumors[2].
Structural highlightsTGFBR structure contains a 100-140 residues ligand-binding N-terminal extracellular domain; a transmembrane domain; a 350-400 amino acid cytoplasmic kinase domain; and a C-terminal zona pellucida (ZP) domain of ca 260 residues which has a role in protein polymerization.
| |
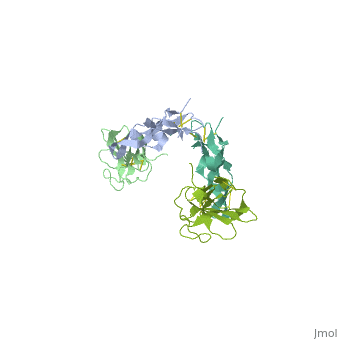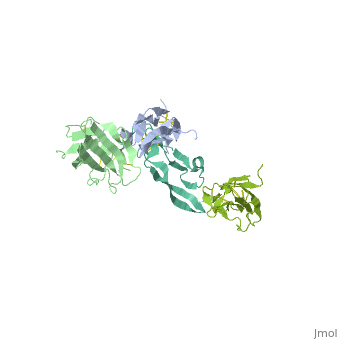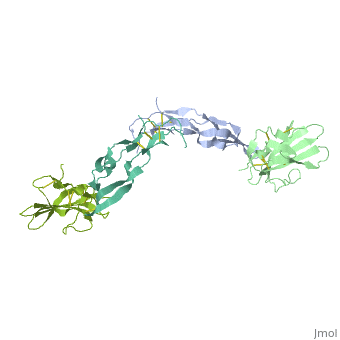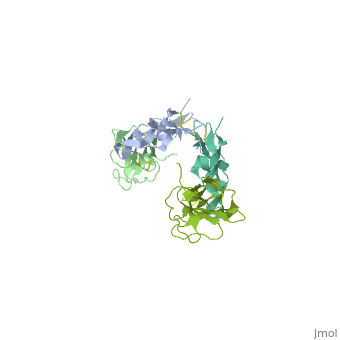
3D Structures of TGF-β receptor3D Structures of TGF-β receptor
Updated on 25-September-2018
{"openlevels":0}
- TGF-β receptor I; kinase domain 200-503
- 1ias, 5e8s – hTGFBR-I kinase domain – human
- 5e8t, 5e8u – hTGFBR-I kinase domain (mutant)
- 5e8w, 5e8x – hTGFBR-I kinase domain (mutant) + staurosporine
- 5e8z – hTGFBR-I kinase domain (mutant) + inhibitor
- 2l5s – hTGFBR-I extracellular domain - NMR
- 1b6c – hTGFBR-I kinase domain + FKBP12
- 1py5, 3faa, 3gxl, 3hmm, 2wot, 2wou, 3kcf, 2x7o, 3tzm, 4x0m, 4x2j, 4x2k, 4x2n – hTGFBR-I kinase domain + inhibitor
- 1vjy – hTGFBR-I residues 1-303 + inhibitor
- 5usq – hTGFBR-I residues 123-421 + inhibitor
- 1rw8 – hTGFBR-I truncated kinase domain + inhibitor
- TGF-β receptor II
- 1m9z – hTGFBR-II extracellular domain
- 1plo, 4p7u – hTGFBR-II extracellular domain (mutant) - NMR
- 5e8v – hTGFBR-II kinase domain (mutant)
- 5e8y – hTGFBR-II kinase domain (mutant) + staurosporine
- 5e92 – hTGFBR-II kinase domain (mutant) + AMPPNP
- 1ks6 – cTGFBR-II extracellular domain - chicken
- 1ktz – hTGFBR-II extracellular domain + TGF-β3
- 5ty4 – hTGFBR-II extracellular domain + mmTGF-β2
- 5tx4 – mTGFBR-II extracellular domain (mutant) + hTGF-β2 - mouse
- TGF-β receptor III
- 3qw9 – TGFBR-III ZP-C domain - rat
- 4ajv – mTGFBR-III ZP-C domain
- TGF-β receptor I+II
- 2pjy – hTGFBR-I extracellular domain (mutant) + hTGFBR-II extracellular domain (mutant) + TGF-β3
- 3kfd – hTGFBR-I extracellular domain + hTGFBR-II extracellular domain + TGF-β1
ReferencesReferences
- ↑ Wrana JL. TGF-beta receptors and signalling mechanisms. Miner Electrolyte Metab. 1998;24(2-3):120-30. PMID:9525694
- ↑ Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood RA, Dietz HC. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013 Jul 24;5(195):195ra94. doi: 10.1126/scitranslmed.3006448. PMID:23884466 doi:http://dx.doi.org/10.1126/scitranslmed.3006448